Abstract
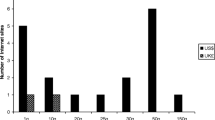
Background Permanently recalled drugs are a public health concern if they remain accessible in violation of applicable regulation. Illicit online pharmacies act as an alternative form of access and have been associated with the sale to patients of counterfeit/falsified/fraudulent/substandard drugs. We wished to determine if permanently recalled and significantly restricted drugs were illegally marketed for sale online. Objective The study was conducted in two phases with two objectives. The first phase attempted to identify drugs subject to permanent recall in certain major pharmaceutical markets as well as those listed as recalled or significantly restricted by the United Nations. We also examined the market authorization status of identified drugs in China and India. The second phase used structured searches on the Internet to determine if identified drugs were marketed for sale online. Setting The World Wide Web. Method After identification of permanently recalled and restricted drugs we conducted Internet searches for illegal “no prescription” marketing events. We assessed the form of marketing, whether a site offered direct-to-patient sale, use of social media marketing, and the site’s compliance status with external monitoring bodies. Main Outcome Number of recalled drugs marketed as available for purchase on the Internet. Results We identified 16 class I equivalent permanently recalled or restricted drugs, 56.3 % (n = 9) of which maintained market authorization in either China or India. Half (n = 8) were marketed for sale online without a prescription direct-to-patient. Use of social media marketing was mixed, with only 18.8 % (n = 3) of recalled drugs having a presence on Facebook, though 50.0 % (n = 8) had content on Twitter. We also found the majority (68.8 %, n = 11) were available and marketed for sale by vendors on the wholesale/business-to-business website alibaba.com primarily as active pharmaceutical ingredient. Conclusion Despite efforts in several countries to restrict access to these drugs or permanently remove them from the market, our study indicates that various sources actively market recalled drugs for sale online. Drug regulators, public health agencies, and law enforcement officials should act with urgency to appropriately restrict and regulate these sales to protect global patients and consumers.

Similar content being viewed by others
References
U.S. Food and Drug Administration [Internet]. FDA Website. [cited 2015 Jun 1]. Drug recalls. http://www.fda.gov/drugs/drugsafety/DrugRecalls/default.htm.
Waxman HA. The lessons of Vioxx—drug safety and sales. N Engl J Med. 2005;352(25):2576–8.
Moore TJ, Psaty BM, Furberg CD. Time to act on drug safety. JAMA. 1998;279(19):1571–3.
Dieppe PA. Lessons from the withdrawal of rofecoxib. BMJ. 2004;329(7471):867–8.
Maxwell SRJ, Webb DJ. Internet pharmacy: A web of mistrust? Br J Clin Pharmacol. 2008;66(2):196–8.
Pharma&MedTech [Internet]. Drug recalls soared again in 2013, driven by contamination. [cited 2015 Jun 1]. http://www.pharmamedtechbi.com/publications/the-pink-sheet/76/26/drug-recalls-soared-again-in-2013-driven-by-contamination.
Mitka M. Improving drug safety. JAMA. 2010;304(10):1060.
Lexchin J. How safer are new drugs? Market withdrawal of drugs approved in Canada between 1990 and 2009. Open Med. 2014;8(1):e14–9.
Almuzaini T, Choonara I, Sammons H. Substandard and counterfeit medicines: a systematic review of the literature. BMJ Open. 2013;3(8):e002923–3.
Williams G. Withdrawal of sibutramine in Europe. BMJ. 2010;340:c824.
Balakrishnan K, Tordoff J, Norris P, Reith D. Establishing a baseline for the monitoring of medicines availability for children in the UK: 1998–2002. Br J Clin Pharmacol. 2007;63(1):85–91.
Mackey TK, Liang BA. The global counterfeit drug trade: patient safety and public health risks. J Pharm Sci. 2011;100(11):4571–9.
Liang BA, Mackey T. Searching for safety: addressing search engine, website, and provider accountability for illicit online drug sales. Am J Law Med. 2009;35(1):125–84.
Liang BA, Mackey TK. Online availability and safety of drugs in shortage: a descriptive study of Internet vendor characteristics. J Med Internet Res. 2012;14(1):e27.
Siva N. Tackling the booming trade in counterfeit drugs. Lancet. 2010;376(9754):1725–6.
Growing threat from counterfeit medicines. Bull World Health Organ. 2010;88(24):247–8.
Fittler A, Bősze G, Botz L. Evaluating aspects of online medication safety in long-term follow-up of 136 Internet pharmacies: illegal rogue online pharmacies flourish and are long-lived. J Med Internet Res. 2013;15(9):e199.
Liang BA, Mackey TK. Prevalence and global health implications of social media in direct-to-consumer drug advertising. J Med Internet Res. 2011;13(3):e64.
Liang BA, Mackey T. Direct-to-consumer advertising with interactive Internet media: global regulation and public health issues. JAMA. 2011;305(8):824–5.
Mackey TK, Liang BA. Pharmaceutical digital marketing and governance: illicit actors and challenges to global patient safety and public health. Global Health. 2013;9(1):45.
Alwon BM, Solomon G, Hussain F, Wright DJ. A detailed analysis of online pharmacy characteristics to inform safe usage by patients. Int J Clin Pharm. 2015;37:148–58.
Liang BA, Mackey TK, Lovett KM. Suspect online sellers and contraceptive access. Contraception. 2012;86(5):551–6.
Liang BA, Mackey TK. Vaccine shortages and suspect online pharmacy sellers. Vaccine. 2012;30(2):105–8.
Khan MH, Tanimoto T, Nakanishi Y, Yoshida N, Tsuboi H, Kimura K. Public health concerns for anti-obesity medicines imported for personal use through the Internet: a cross-sectional study. BMJ Open. 2012;2(3):e000854–4.
Liang BA, Mackey TK. Sexual medicine: online risks to health—the problem of counterfeit drugs. Nat Rev Urol. 2012;9(9):480–2.
Lovett KM, Liang BA, Mackey TK. Online, direct-to-consumer access to insulin: patient safety considerations and reform. J Diabetes Sci Technol. 2012;6(6):1503–6.
Liang BA, Mackey TK, Lovett K. Emerging dangers from direct botulinum access and use. J Homel Secur Emerg Manag. 2012;. doi:10.1515/1547-7355.1973.
Forman RF. Availability of opioids on the Internet. JAMA. 2003;290(7):889.
Forman RF, Block LG. The marketing of opioid medications without prescription over the Internet. J Pub Policy Mark. 2006;25:133–46.
Jena AB, Goldman DP, Foster SE, Califano JA. Prescription medication abuse and illegitimate Internet-based pharmacies. Ann Intern Med. 2011;155(12):848–50.
Lagan BM, Dolk H, White B, Uges DRA, Sinclair M. Assessing the availability of the teratogenic drug isotretinoin outside the pregnancy prevention programme: a survey of e-pharmacies. Pharmacoepidemiol Drug Saf. 2014;4:411–8.
Tatsioni A, Gerasi E, Charitidou E, Simou N, Mavreas V, Ioannidis JPA. Important drug safety information on the Internet: assessing its accuracy and reliability. Drug Saf. 2003;26(7):519.
Liang BA, Mackey TK, Lovett KM. Illegal “no prescription” Internet access to narrow therapeutic index drugs. Clin Ther. 2013;35(5):694–700.
Liang BA, Mackey TK, Archer-Hayes AN, Shinn LM. Illicit online marketing of locaserin before DEA scheduling. Obesity. 2013;21(5):861–4.
National Association of Boards of Pharmacy [Internet]. Not recommended sites. [cited 2015 Jun 1]. http://www.nabp.net/programs/consumer-protection/buying-medicine-online/not-recommended-sites/.
LegitScript. [Internet]. Frequently asked questions. [cited 2015 Jun 1]. http://www.legitscript.com/about/faq.
Ninan B, Wertheimer AI. Withdrawing drugs in the us versus other countries. Innov Pharm. 2012;3(3):1–12.
Wolfe SM. When EMA and FDA decisions conflict: Differences in patients or in regulation? BMJ. 2013;347:f5140.
Tafuri G, Stolk P, Trotta F, Putzeist M, Leufkens HG, Laing RO, et al. How do the EMA and FDA decide which anticancer drugs make it to the market? A comparative qualitative study on decision makers’ views. Ann Oncol. 2014;25(1):265–9.
Wang B, Gagne JJ, Choudhry NK. The epidemiology of drug recalls in the United States. Arch Intern Med. 2012;172(14):1110–1.
Acknowledgments
PA received support from an NIH Summer Research Training Grant Fellowship and greatly acknowledges this support. The authors would like to thank the Canadian Social Sciences and Humanities Research council for their support.
Funding
TM received funding from the Canadian Social Sciences and Humanities Research Council in support of this research.
Conflicts of interest
The authors have report no conflicts of interest pertaining to this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mackey, T.K., Aung, P. & Liang, B.A. Illicit Internet availability of drugs subject to recall and patient safety consequences. Int J Clin Pharm 37, 1076–1085 (2015). https://doi.org/10.1007/s11096-015-0154-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-015-0154-8