Abstract
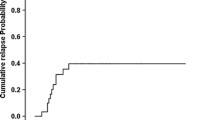
Background Busulfan/cyclophosphamide (Bu/Cy) is commonly used as a standard conditioning regimen without total body irradiation for patients with hematological myeloid malignancies undergoing hematopoietic stem cell transplantation (HSCT). Objective To develop a new myeloablative conditioning regimen incorporating fludarabine (Flu) and cytarabine (Ara-c). Setting A tertiary blood disease hospital in Tianjin, China. Methods A Bu/Cy preparative regimen was used, modified by Flu 90 mg/m2 and Ara-c 6 g/m2 in 57 unselected patients (median age 37 years) with hematological myeloid malignancies. The patients were to receive allogeneic hematopoietic stem cell transplantation (allo-HSCT). Thirteen patients had high-risk leukemia, fifty patients had HLA matched sibling donors while seven patients had HLA mismatched sibling donors. Cy was given 50 mg/kg/day for 2 days while Bu was given 3.2 mg/kg/day intravenously for 3 days. Main outcome measure Post-transplant donor chimerism, relapse tendency and minimal residual disease. Results Extramedullar toxicity was relatively limited; the incidence of treatment-related mortality (TRM) within 100 days was 3.5 %. The incidence of grade II–IV, grade III–IV acute graft versus host disease (GVHD) and chronic GVHD of the evaluable patients were 21.1, 8.8 and 36.4 %, respectively. With a median follow up of 59 (13–96.5) months, TRM and relapse rate (RR) at eight years were 24.1 ± 5.8 and 14.7 ± 4.8 %, respectively. Disease free survival at eight years was 67.9 ± 6.2 % for the entire group, 60.0 ± 8.9 % for patients with AML, 77.3 ± 8.9 % for patients with CML, 70.0 ± 6.5 and 42.9 ± 18.7 % or matched sibling and mismatched sibling HSCT respectively. Conclusion The new regimen was associated with a low relapse rate, low incidence and severity of graft versus host disease and satisfactory survival for patients with myeloid malignancies.



Similar content being viewed by others
References
McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–8.
Ringdén O, Remberger M, Ruutu T, Nikoskelainen J, Volin L, Vindeløv L, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–201.
Hassan M, Ljungman P, Ringdén O, Hassan Z, Oberg G, Nilsson C, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915–24.
Ringdén O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindeløv L, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–30.
Kerbauy FR, Tirapelli B, Akabane H, Oliveira JS. The effect of administration order of BU and CY on toxicity in hematopoietic SCT in humans. Bone Marrow Transplant. 2009;43:883–5.
Sadeghi B, Jansson M, Hassan Z, Mints M, Hägglund H, Abedi-Valugerdi M, et al. The effect of administration order of BU and CY on engraftment and toxicity in HSCT mouse model. Bone Marrow Transplant. 2008;41:895–904.
Cantoni N, Gerull S, Heim D, Halter J, Bucher C, Buser A, et al. Order of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:344–9.
Kashyap A, Wingard J, Cagnoni P, Jones R, Tarantolo S, Hu W, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500.
Ferry C, Socié G. Busulfan-cyclophosphamide versus total body irradiation-cyclophosphamide as preparative regimen before allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia: what have we learned? Exp Hematol. 2003;31:1182–6.
Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen–a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:3549–56.
Clift RA, Buckner CD, Thomas ED, Bensinger WI, Bowden R, Bryant E, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84:2036–43.
Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79:2578–82.
Devergie A, Blaise D, Attal M, Tigaud JD, Jouet JP, Vernant JP, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM). Blood. 1995;85:2263–8.
Estey E, Plunkett W, Gandhi V, Rios MB, Kantarjian H, Keating MJ. Fludarabine and arabinosylcytosine therapy of refractory and relapsed acute myelogenous leukemia. Leuk Lymphoma. 1993;9:343–50.
Tedeschi A, Montillo M, Ferrara F, Nosari A, Mele G, Copia C, et al. Treatment of chronic myeloid leukemia in the blastic phase with fludarabine, cytosine arabinoside and G-CSF (FLAG). Eur J Haematol. 2000;64:182–7.
Pawson R, Potter MN, Theocharous P, Lawler M, Garg M, Yin JA, et al. Treatment of relapse after allogeneic bone marrow transplantation with reduced intensity conditioning (FLAG ± Ida) and second allogeneic stem cell transplant. Br J Haematol. 2001;115:622–9.
Okada M, Fujimori Y, Misawa M, Kai S, Nakajima T, Okikawa Y, Satake A, et al. Unrelated umbilical cord blood transplantation using a TBI/FLAG conditioning regimen for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2008;14:896–903.
US National Cancer Institute. Cancer therapy evaluation program common toxicity criteria, version 2.0. Bethesda, MD: DCTD, NCI, NIH, DHHS; 1998.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9.
Iravani M, Evazi MR, Mousavi SA, Shamshiri AR, Tavakoli M, Ashouri A, et al. Fludarabine and busulfan as a myeloablative conditioning regimen for allogeneic stem cell transplantation in high-and standard-risk leukemic patients. Bone Marrow Transplant. 2007;40:105–10.
Chunduri S, Dobogai LC, Peace D, Saunthararajah Y, Quigley J, Chen YH, et al. Fludarabine/i.v. BU conditioning regimen: myeloablative, reduced intensity or both? Bone Marrow Transplant. 2008;41:935–40.
Chae YS, Sohn SK, Kim JG, Cho YY, Moon JH, Shin HJ, et al. New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation: comparison with BuCy2. Bone Marrow Transplant. 2007;40:541–7.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–76.
Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820–6.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–64.
Santarone S, Pidala J, Di Nicola M, Field T, Alsina M, Ayala E, et al. Fludarabine and pharmacokinetic-targeted busulfan before allografting for adults with acute lymphoid leukemia. Biol Blood Marrow Transplant. 2011;17(10):1505–11.
Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2013;20(3):345–53.
Kumar M, Saleh A, Rao PV, Ochoa S, Meyers L, Miller A, et al. Toxicity associated with high-dose cytosine arabinoside and total body irradiation as conditioning for allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19:1061–4.
Champlin R, Jacobs A, Gale RP, Ho W, Selch M, Lenarsky C, et al. High-dose cytarabine in consolidation chemotherapy or with bone marrow transplantation for patients with acute leukemia: preliminary results. Semin Oncol. 1985;12(Suppl. 3):190–5.
Weyman C, Graham-Pole J, Emerson S, August C, Champlin R, Coccia P, et al. Use of cytosine arabinoside and total body irradiation as conditioning for allogeneic marrow transplantation in patients with acute lymphoblastic leukemia: a multicenter survey. Bone Marrow Transplant. 1993;11:43–50.
Mineishi S, Longo WL, Atkinson ME, Smith EP, Hamielec M, Wiersma SR, et al. Addition of high-dose Ara-C to the BMT conditioning regimen reduces leukemia relapse without an increase in toxicity. Bone Marrow Transplant. 1999;23:1217–22.
Gordon BG, Warkentin PI, Strandjord SE, Abromowitch M, Bayever E, Harper JL, et al. Allogeneic bone marrow transplantation for children with acute leukemia: long-term follow-up of patients prepared with high-dose cytosine arabinoside and fractionated total body irradiation. Bone Marrow Transplant. 1997;20:5–10.
Gandhi V, Plunkett W. Clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41:93–103.
Ringdén O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;26:3110–8.
Radich JP, Gooley T, Bensinger W, Chauncey T, Clift R, Flowers M, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003;102:31–5.
Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D, et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–8.
Martino R, Pérez-Simón JA, Moreno E, Queraltó JM, Caballero D, Mateos M, et al. Reduced-intensity conditioning allogeneic blood stem cell transplantation with fludarabine and oral busulfan with or without pharmacokinetically targeted busulfan dosing in patients with myeloid leukemia ineligible for conventional conditioning. Biol Blood Marrow Transplant. 2005;11:437–47.
Funding
This work was supported by Grants from the National Key Technology Support Program of China (2013BAIO1BO9) and the Key Program of Applied basic Research Foundation of Tianjin (14JCZDJC33000).
Conflicts of interest
The authors have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, X., Wei, J., He, Y. et al. A modified busulfan and cyclophosphamide preparative regimen for allogeneic transplantation in myeloid malignancies. Int J Clin Pharm 37, 44–52 (2015). https://doi.org/10.1007/s11096-014-0036-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-014-0036-5