Abstract
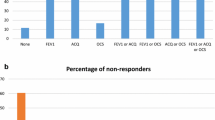
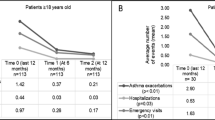
Background Omalizumab was introduced in Malta in 2011. To date, no local data have been published. Objective To obtain baseline characteristics of our local cohort, determine effectiveness of omalizumab at 52 weeks, compare clinical outcomes 52 weeks pre- and postomalizumab therapy and to assess its safety and tolerability. Setting The Mater Dei Hospital in Malta. Method All consented adult patients who were eligible to start treatment with omalizumab for asthma were enrolled in this open, prospective observational real-life study. A questionnaire was completed and an Asthma Control Test and spirometry performed. Patients were reviewed on a regular basis. Any undesirable symptoms were recorded. Treatment effectiveness was evaluated at 16 and 52 weeks, during which a decision was taken whether patients were responders. Outcomes were compared 52 weeks pre- and post- treatment initiation. Main outcome measure To determine effectiveness of treatment following 1 year of omalizumab by assessing its impact on the rate of asthma-related exacerbations and health care utilization including hospitalizations. Results Our cohort included 22 patients, all non-smokers (mean age 52.7 ± 11, 64 % males). The mean baseline IgE level was 448.6 ± 444 IU/ml. At week 12, treatment was stopped in one patient due to arthralgias. The drug was stopped in two patients at week 16 due to treatment ineffectiveness. At week 20, treatment was stopped in another patient in view of arthralgias. A significant reduction in the number of asthma exacerbations (p = .03) and number of systemic steroid courses required (p = .03) was identified at 52 weeks. There was a significant improvement in the ACT score (p < .001) after 52 weeks but no significant improvement in FEV1. There was a non-significant decline in the number of hospitalizations (p = .6), asthma-related healthcare visits (p = .2) and days off work (p = .09). Adverse events occurred in 10 % of patients. Costs related to asthma hospital-stay and medicines administered during hospitalisations were decreased by half following 1 year on omalizumab. Conclusion Omalizumab treatment resulted in an improved asthma control, with a significant reduction in asthma exacerbations and systemic steroid courses required and improvement on ACT score. Adverse events were infrequent and the drug was well tolerated.


Similar content being viewed by others
References
Braunstahl G, Chen C-W, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107(8):1141–51.
From the Global Strategy for Asthma Prevention, Global Initiative for Asthma (GINA). 2010. http://www.ginasthma.org. Accessed 15 Jan 2014.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343.
Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–82.
Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–90.
Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–16.
Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66(5):671–8.
Holgate ST, Chuchalin AG, Hebert J, Lötvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34(4):632–8.
Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124(6):1210–6.
Barnes N, Menzies-Gow A, Mansur A, Spencer D, Percival F, Radwan R, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real world study. J Asthma. 2013;50(5):529–36.
Schumann C, Kropf C, Wibmer T, Rüdiger S, Stoiber KM, Thielen A, et al. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J. 2012;6(4):215–27.
Brusselle G, Michils A, Louis R, Dupont L, Van de Maele B, Delobbe A, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103:1633–42.
Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–31.
Cazzola M, Camiciottoli G, Bonavia M, Gulotta C, Ravazzi A, Alessandrini A, et al. Italian real-life experience of omalizumab. Respir Med. 2010;104:1410–6.
Korn S, Schumann C, Kropf C, Stoiber K, Thielen A, Taube C, et al. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010;105(4):313–9.
Grimaldi-Bensouda L, Zureik M, Aubier M, Humbert M, Levy J, Benichou J, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143(2):398–405.
European Medicines Agency (EMEA) Omalizumab (Xolair®) full prescribing information (EU). 2007. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000606/human_med_001162.jsp&mid=WC0b01ac058001d124. 6 Aug 2013.
Rottem M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: real-life experience in Israel. J Asthma. 2012;49:78–82.
Summary of Product Characteristics Omalizumab (Xolair®). 2014. http://www.medicines.org.uk/emc/medicine/24912/SPC/Xolair+150mg+Solution+for+InjectIion.
Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65.
Lloyd A, Turk F, Leighton T, Canonica GW. Psychometric evaluation of global evaluation of treatment effectiveness: a tool to assess patients with moderate-to-severe allergic asthma. J Med Econ. 2007;10(3):285–96.
Thomas M, Kay S, Pike J, Williams A, Rosenzweig JR, Hillyer EV, et al. The Asthma Control Test (ACT) as a predictor of GINA-guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009;18:41–9.
Malta Government Gazzette. Healthcare fees regulations. Legal Notice 201 of 2004.
British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary. Great Britain: The Pharmaceutical Press; 2014.
Health care in Malta, hospitals, health centres and pharmacies. 2011. http://www.malta.com/en/local-information/health-care. 2 Mar 2014.
National Statistics office Labour force survey: Q2/2013. 2013. http://nso.gov.mt/statdoc/document_file.aspx?id=3722. 2 Mar 2014.
National Institute for Health and Clinical Excellence. Asthma (severe, persistent, patients aged 6 + , adults)—omalizumab (rev TA133, TA201): appraisal consultation document. 2012. http://guidance.nice.org.uk/TA/WaveR/110/Consultation/DraftGuidance. 26 Jan 2013.
National Institute for Health and Clinical Excellence. Omalizumab for treating severe persistent allergic asthma (review of technology appraisal guidance 133 and 201). 2013. http://www.nice.org.uk/nicemedia/live/13550/62987.pdf. 29 Jul 2013.
Molimard M, de Blay F, Didier A, Le Gros V. Effetiveness of omalizumab (Xolair®) in the first patients treated in real-life practice in France. Respir Med. 2008;102:71–6.
Herland K, Akselsen JP, Skjønsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99(1):11–9.
Vieira T, de Oliveira JF, da Graca Castel-Branco M. Short and long-term quality of life and asthma control with omalizumab therapy in a real life setting in Portugal. Allergol Immunopathol (Madr). 2012; doi:10.1016/j.aller.2012.07.006. 28 Nov 2013.
Tzortzaki EG, Georgiou A, Kampas D, Lemessios M, Markatos M, Adamidi T, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther. 2012;25(1):77–82.
Menzella F, Facciolongo N, Piro R, Formisano D, Roggeri A, Simonazzi A, et al. Clinical and pharmacoeconomic aspects of omalizumab: a 4-year follow-up. Ther Adv Respir Dis. 2012;6(2):87–95.
Funding
None.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gouder, C., West, L.M. & Montefort, S. The real-life clinical effects of 52 weeks of omalizumab therapy for severe persistent allergic asthma. Int J Clin Pharm 37, 36–43 (2015). https://doi.org/10.1007/s11096-014-0034-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-014-0034-7