ABSTRACT
Purpose
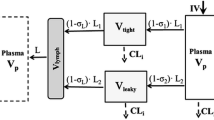
To examine the across-species scalability of monoclonal antibody (mAb) pharmacokinetics (PK) and assess similarities in tissue distribution across species using a recently developed minimal PBPK (mPBPK) model.
Methods
Twelve sets of antibody PK data from various species were obtained from the literature, which were jointly and individually analyzed. In joint analysis, vascular reflection coefficients for tissues with either tight (σ 1 ) or leaky endothelium (σ 2 ) were assumed consistent across species with systemic clearance allometrically scaled (CL = a∙BW b). Four parameters (σ 1 , σ 2 , a, and b) were estimated in the joint analysis. In addition, the PK from each species was individually analyzed to assess species similarities in tissue distribution.
Results
Twelve mAb PK profiles were well-captured by the mPBPK model in the joint analysis. The estimated σ 1 ranged 0.690 to 0.999 with an average of 0.908; and σ 2 ranged 0.258 to 0.841 with an average of 0.579. Clearance was reasonably scaled and b ranged 0.695 to 1.27 averaging 0.91. Predictions of plasma concentrations for erlizumab and canakinumab in humans using parameters obtained from fitting animal data were consistent with actual measurements.
Conclusions
Therapeutic mAbs given IV usually exhibit biexponential kinetics with their distribution properties best captured using physiological concepts. The mPBPK modeling approach may facilitate efforts in translating antibody distribution and overall PK across species.








Similar content being viewed by others
Abbreviations
- AIC:
-
Akaike Information Criterion
- AUC:
-
Area under the plasma concentration-time curve
- BW:
-
Body weight
- CV:
-
Coefficient of variation
- FIH:
-
First-in-human
- ISF:
-
Interstitial fluid
- L:
-
Lymph flow
- mAb:
-
Monoclonal antibody
- mPBPK:
-
Minimal physiologically based pharmacokinetic modeling
- NCA:
-
Non-compartmental analysis
- PBPK:
-
Physiologically based pharmacokinetic modeling
- PK:
-
Pharmacokinetic
- SC:
-
Schwarz Criterion
REFERENCES
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3(1):61–6.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58.
Mordenti J. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci. 1986;75(11):1028–40.
Wang W, Prueksaritanont T. Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Biopharm Drug Dispos. 2010;31(4):253–63.
Ritschel WA, Vachharajani NN, Johnson RD, Hussain AS. The allometric approach for interspecies scaling of pharmacokinetic parameters. Comp Biochem Physiol C Comp Pharmacol Toxicol. 1992;103(2):249–53.
Duconge J, Fernandez-Sanchez E, Alvarez D. Interspecies scaling of the monoclonal anti-EGF receptor ior EGF/r3 antibody disposition using allometric paradigm: is it really suitable? Biopharm Drug Dispos. 2004;25(4):177–86.
Boxenbaum H, Fertig JB. Scaling of antipyrine intrinsic clearance of unbound drug in 15 mammalian species. Eur J Drug Metab Pharmacokinet. 1984;9(2):177–83.
Mahmood I, Balian JD. Interspecies scaling: predicting clearance of drugs in humans. Three different approaches. Xenobiotica; the fate of foreign compounds in biological systems. 1996;26(9):887–95.
Lave T, Coassolo P, Reigner B. Prediction of hepatic metabolic clearance based on interspecies allometric scaling techniques and in vitro-in vivo correlations. Clin Pharmacokinet. 1999;36(3):211–31.
Bischoff KB, Dedrick RL, Zaharko DS. Preliminary model for methotrexate pharmacokinetics. J Pharm Sci. 1970;59(2):149–54.
Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54(6):1517–28.
Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK. Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 1995;55(20):4611–22.
Davda JP, Jain M, Batra SK, Gwilt PR, Robinson DH. A physiologically based pharmacokinetic (PBPK) model to characterize and predict the disposition of monoclonal antibody CC49 and its single chain Fv constructs. Int Immunopharmacol. 2008;8(3):401–13.
Mann S, Droz PO, Vahter M. A physiologically based pharmacokinetic model for arsenic exposure. II. Validation and application in humans. Toxicol Appl Pharmacol. 1996;140(2):471–86.
Cao Y, Balthasar JP, Jusko WJ. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2013;40(5):597–607.
Cao Y, Jusko WJ. Survey of monoclonal antibody disposition in man utilizing a minimal physiologically-based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2014;41(6):571–80.
Cao Y, Jusko WJ. Incorporating target-mediated drug disposition in a minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2014;41(4):375–87.
Pang KS, Rowland M. Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J Pharmacokinet Biopharm. 1977;5(6):625–53.
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39(1):67–86.
Rossing N. Intra- and extravascular distribution of albumin and immunoglobulin in man. Lymphology. 1978;11(4):138–42.
Boxenbaum H. Interspecies pharmacokinetic scaling and the evolutionary-comparative paradigm. Drug Metab Rev. 1984;15(5-6):1071–121.
Porter CJ, Edwards GA, Charman SA. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv Drug Deliv Rev. 2001;50(1-2):157–71.
Lindena J, Kupper W, Trautschold I. Catalytic enzyme activity concentration in thoracic duct, liver, and intestinal lymph of the dog, the rabbit, the rat and the mouse. Approach to a quantitative diagnostic enzymology, II. Communication. J Clin Chem Clin Biochem. 1986;24(1):19–33.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Rodionov N. Graph digitizer version 1.9. 2000.
D’Argenio DZ, Schumitzky A. ADAPT V user’s guide: pharmacokinetic/pharmacodynamic systerm analysis software. Los Angeles: Biomedical Simulations Resorce; 2009.
Vugmeyster Y, Szklut P, Tchistiakova L, Abraham W, Kasaian M, Xu X. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of humanized monoclonal anti-IL-13 antibodies with different IL-13 neutralization mechanisms. Int Immunopharmacol. 2008;8(3):477–83.
Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, et al. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288(1):371–8.
Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34(5):687–709.
Tang H, Mayersohn M. A novel model for prediction of human drug clearance by allometric scaling. Drug Metab Dispos. 2005;33(9):1297–303.
Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol. 2009;49(12):1382–402.
Mordenti J, Chen SA, Moore JA, Ferraiolo BL, Green JD. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm Res. 1991;8(11):1351–9.
Bumbaca D, Boswell CA, Fielder PJ, Khawli LA. Physiochemical and biochemical factors influencing the pharmacokinetics of antibody therapeutics. AAPS J. 2012;14(3):554–8.
Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem. 2010;21(12):2153–63.
Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009;8(10):2861–71.
ACKNOWLEDGMENTS AND DISCLOSURES
This research was supported by the National Institutes of Health Grant GM57980 and the UB Center for Protein Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Also provided are further references pertaining to the specific antibodies that were modeled and the Adapt computer code used for joint modeling of mAb profiles from two or more species.
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Cao, Y. & Jusko, W.J. Across-Species Scaling of Monoclonal Antibody Pharmacokinetics Using a Minimal PBPK Model. Pharm Res 32, 3269–3281 (2015). https://doi.org/10.1007/s11095-015-1703-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1703-5