Abstract
Purpose
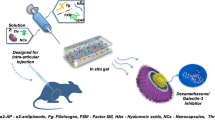
Hyaluronic acid (HA) is an imperative biomaterial with desirable rheological properties to alleviate symptoms of osteoarthritis. Nevertheless, scantly percutaeous permeation of this macromolecule handicaps its effective use for orthopedics and triggers intra-articular injection as the only surrogate. This study presents novel self-assembeld HA-based gel core elastic nanovesicles, (hyaluosomes; GC-HS), for non-invasive transdermal delivery of HA.
Methods
GC-HS were prepared with 1% HA using simple film hydration technique. Their size, zeta potential, percentage entrapment efficiency (% EE), elasticity, and ex-vivo transdermal permeation were evaluated compared to conventional liposomes CL. Structure elucidation of the formed novel system was performed using light, polarizing and transmission electron microscopy. In-vivo permeation of GC-HS through knee joints of female Sprague Dawley rats was compared to CL and HA alone.
Results
GC-HS showed nanosize (232.8 ± 7.2), high negative zeta potential (−45.1 ± 8.3) and higher elasticity (size alteration 5.43%) compared to CL. This novel system has self-penetration enhancing properties compared to CL and plain gel. GC-HS showed self-assembled properties and high physically stable for at least 6 months at 4°C. Ex-vivo permeation of HS was significantly higher than CL and plain HA gel alone. In-vivo study exhibited significant six folds increase in transdermal permeation of HA to knee joints from GC-HS compared to plain HA gel.
Conclusion
Novel GC-HS are promising nanogels for topical management of osteoarthritis surrogating the need for intra-articular injection.








Similar content being viewed by others
Abbreviations
- CL:
-
Conventional liposomes
- CLG:
-
Conventional liposomal gel
- GC-HS:
-
Gel core hyaluosomes
- HA:
-
Hyaluronic acid
- HS:
-
Hyaluosomes
- SC:
-
Stratum corneum
References
Rachakonda VK, Yerramsetty KM, Madihally SV, Robinson Jr RL, Gasem KA. Screening of chemical penetration enhancers for transdermal drug delivery using electrical resistance of skin. Pharm Res. 2008;25(11):2697–704.
Elnaggar YS, El-Massik MA, Abdallah OY. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int J Nanomedicine. 2011;6:3195–205.
Elnaggar YS, El-Massik MA, Abdallah OY. Sildenafil citrate nanoemulsion vs. self-nanoemulsifying delivery systems: rational development and transdermal permeation. Int J Nanotechnol. 2011;8(8):749–63.
Elnaggar YS, El-Refaie WM, El-Massik MA, Abdallah OY. Lecithin-based nanostructured gels for skin delivery: an update on state of art and recent applications. J Control Release. 2014;180:10–24.
Vyas LK, Tapar KK, Nema RK, Parashar AK. Development and characterization of topical liposomal gel formulation for anti-cellulite activity. Int J Pharm Pharm Sci. 2013;5(3):512–6.
Nikalje A, Tiwari S. Ethosomes: a novel tool for transdermal drug delivery. Indian J Pharm Sci. 2012;2(1):1–20.
Bragagni M, Mennini N, Maestrelli F, Cirri M, Mura P. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Deliv. 2012;19(7):354–61.
Elsayed M, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006;322(1):60–6.
Hiruta Y, Hattori Y, Kawano K, Obata Y, Maitani Y. Novel ultra-deformable vesicles entrapped with bleomycin and enhanced to penetrate rat skin. J Control Release. 2006;113(2):146–54.
Malakar J, Sen SO, Nayak AK, Sen KK. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm J. 2012;20(4):355–63.
Gupta A, Aggarwal G, Singla S, Arora R. Transfersomes: a novel vesicular carrier for enhanced Transdermal delivery of sertraline: development, characterization, and performance evaluation. Sci Pharm. 2012;80(4):1061–80.
Touitou E, Godin B. Enhanced skin permeation using ethosomes. In: Smith EMH, editor. Percutaneous penetration enhancers. 2nd ed. New York: CRC Press; 2005.
Bahia APC, Azevedo EG, Ferreira LA, Frézard F. New insights into the mode of action of ultradeformable vesicles using calcein as hydrophilic fluorescent marker. Eur J Pharm Sci. 2010;39(1):90–6.
Helwa Y, Dave N, Liu J. Electrostatically directed liposome adsorption, internalization and fusion on hydrogel microparticles. Soft Matter. 2013;9:6151–8.
An E, Jeong CB, Cha C, Kim DH, Lee H, Kong H, et al. Fabrication of microgel-in-liposome particles with improved water retention. Langmuir. 2012;28(9):4095–101.
Tiwari S, Goyal AK, Khatri K, Mishra N, Vyas SP. Gel core liposomes: an advanced carrier for improved vaccine delivery. J Microencapsul. 2009;26(1):75–82.
Tiwari S, Goyal AK, Mishra N, Khatri K, Vaidya B, Mehta A, et al. Development and characterization of novel carrier gel core liposomes based transmission blocking malaria vaccine. J Control Release. 2009;140(2):157–65.
Robert L, Robert A, Renard G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol Biol. 2010;58(3):187–98.
Witteveen AG, Sierevelt IN, Blankevoort L, Kerkhoffs GM, van Dijk CN. Intra-articular sodium hyaluronate injections in the osteoarthritic ankle joint: effects, safety and dose dependency. Foot Ankle Surg. 2010;16(4):159–63.
Salk RS, Chang TJ, D’Costa WF, Soomekh DJ, Grogan KA. Sodium hyaluronate in the treatment of osteoarthritis of the ankle: a controlled, randomized, double-blind pilot study. J Bone Joint Surg. 2006;88(2):295–302.
Kong M, Chen XG, Kweon DK, Park HJ. Investigations on skin permeation of hyaluronic acid based nanoemulsion as transdermal carrier. Carbohydr Polym. 2011;86(2):837–43.
Kong M, Park HJ. Stability investigation of hyaluronic acid based nanoemulsion and its potential as transdermal carrier. Carbohydr Polym. 2011;83(3):1303–10.
Brown M, Jones SA. Hyaluronic acid: a unique topical vehicle for the localized delivery of drugs to the skin. J Eur Acad Dermatol Venereol. 2005;19(3):308–18.
Brown TJ, Alcorn D, Fraser JRE. Absorption of hyaluronan applied to the surface of intact skin. J Investig Dermatol. 1999;113(5):740–6.
Kage M, Tokudome Y, Hashimoto F. Permeation of hyaluronan tetrasaccharides through hairless mouse skin: an in vitro and in vivo study. Arch Dermatol Res. 2013;305(1):69–77.
Chen M, Gupta V, Anselmo AC, Muraski JA, Mitragotri S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J Control Release. 2014;173:67–74.
Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9.
Gaafar PM, Abdallah OY, Farid RM, Abdelkader H. Preparation, characterization and evaluation of novel elastic nano-sized niosomes (ethoniosomes) for ocular delivery of prednisolone. J Liposome Res. 2014;24:1–12.
Freag MS, Elnaggar YS, Abdallah OY. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: optimization and ex vivo permeation. Int J Nanomedicine. 2013;8:2385–97.
Freag MS EY, Abdallah OY. Development of novel polymer-stabilized diosmin nanosuspensions: in vitro appraisal and ex vivo permeation. Int J Pharm. 2013;454:642–71.
Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle J-P, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13(9):647–53.
Fagnola M, Pagani MP, Maffioletti S, Tavazzi S, Papagni A. Hyaluronic acid in hydrophilic contact lenses: spectroscopic investigation of the content and release in solution. Cont Lens Anterior Eye. 2009;32(3):108–12.
Saranraj P, Sivakumar S, Sivasubramanian J, Geetha M. Production, optimization and spectroscopic studies of Hyaluronic acid extracted from Streptococcus pyogenes. Int J Pharm Biol Arch. 2011;2(3):954–9.
Kutsch H, Schleich C. Improved colorimetric determination of high-molecular weight hyaluronic acid from synovial fluids. Fresenius’ Z Anal Chem. 1989;333(8):810–7.
Maghraby GME, Williams AC, Barry BW. Skin delivery of oestradiol from deformable and traditiona liposomes: mechanistic studies. J Pharm Pharmacol. 1999;51(10):1123–34.
El Maghraby GM, Williams AC, Barry BW. Oestradiol skin delivery from ultradeformable liposomes: refinement of surfactant concentration. Int J Pharm. 2000;196(1):63–74.
Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta. 1992;1104(1):226–32.
Birkenfeld B, Parafiniuk M, Bielecka-Grzela S, Klimowicz A, Piwowarska-Bilska H, Mikołajczak R, et al. The penetration of topically applied ointment containing hyaluronic acid in rabbit tissues. Pol J Vet Sci. 2011;14(4):621–7.
Kirby C, Gregoriadis G. Dehydration-rehydration vesicles: a simple method for high yield drug entrapment in liposomes. Nat Biotechnol. 1984;2(11):979–84.
Duangjit S, Obata Y, Sano H, Onuki Y, Opanasopit P, Ngawhirunpat T, et al. Comparative study of novel ultradeformable liposomes: menthosomes, transfersomes and liposomes for enhancing skin permeation of meloxicam. Biol Pharm Bull. 2014;37(2):239–47.
Gupta PN, Mishra V, Rawat A, Dubey P, Mahor S, Jain S, et al. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: a comparative study. Int J Pharm. 2005;293(1):73–82.
Fang Y-P, Tsai Y-H, Wu P-C, Huang Y-B. Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int J Pharm. 2008;356(1):144–52.
Lei W, Yu C, Lin H, Zhou X. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J Pharm Sci. 2013;8(6):336–45.
Müller-Goymann C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur J Pharm Biopharm. 2004;58(2):343–56.
Oka T, Miyahara R, Teshigawara T, Watanabe K. Development of novel cosmetic base using sterol surfactant. I. Preparation of novel emulsified particles with sterol surfactant+. J Oleo Sci. 2008;57(10):567–75.
Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Coll Surf B Biointerf. 2003;30(1):129–38.
Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. Int J Pharm. 2007;338(1):317–26.
Conflict of interest
The authors report no financial or personal conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Refaie, W.M., Elnaggar, Y.S.R., El-Massik, M.A. et al. Novel Self-assembled, Gel-core Hyaluosomes for Non-invasive Management of Osteoarthritis: In-vitro Optimization, Ex-vivo and In-vivo Permeation. Pharm Res 32, 2901–2911 (2015). https://doi.org/10.1007/s11095-015-1672-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1672-8