Abstract
Purpose
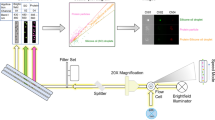
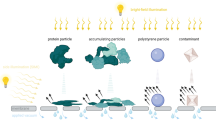
Sub-visible particles were shown to facilitate unwanted immunogenicity of protein therapeutics. To understand the root cause of this phenomenon, a comprehensive analysis of these particles is required. We aimed at establishing a flow-cytometry-based technology to analyze the amount, size distribution and nature of sub-visible particles in protein solutions.
Methods
We adjusted the settings of a BD FACS Canto II by tuning the forward scatter and the side scatter detectors and by using size calibration beads to facilitate the analysis of particles with sizes below 1 μM. We applied a combination of Bis-ANS (4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid dipotassium salt) and DCVJ (9-(2,2-dicyanovinyl)julolidine) to identify specific characteristics of sub-visible particles.
Results
The FACS technology allows the analysis of particles between 0.75 and 10 μm in size, requiring relatively small sample volumes. Protein containing particles can be distinguished from non-protein particles and cross-β-sheet structures contained in protein particles can be identified.
Conclusions
The FACS technology provides robust and reproducible results with respect to number, size distribution and specific characteristics of sub-visible particles between 0.75 and 10 μm in size. Our data for number and size distribution of particles is in good agreement with results obtained with the state-of-the-art technology micro-flow imaging.






Similar content being viewed by others
Abbreviations
- Aβ 1–40:
-
Amyloid beta 1–40 peptide
- Bis-ANS:
-
4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid dipotassium salt
- DCVJ:
-
9-(2,2-dicyanovinyl)julolidine
- DMSO:
-
Dimethyl sulfoxide
- D-PBS:
-
Dulbecco’s Phosphate-Buffered Saline
- preparation Non-Prot:
-
Non-protein particles
- preparation Prot:
-
Protein particles without cross-ß-sheet structures
- preparation Prot-Crossß:
-
Protein particles containing cross-ß-sheet structures
- rFVIII:
-
Recombinant human factor VIII
- TEM:
-
Transmission Electron Microscopy
- ThT:
-
Thioflavin T
- WFI:
-
Water For Injection
References
Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39.
Büttel IC, Chamberlain P, Chowers Y, Ehmann F, Greinacher A, Jefferis R, et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals. 2011;39(2):100–9.
Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself. 2010;1(4):314–22.
Schellekens H. The immunogenicity of therapeutic proteins. Discov Med. 2010;9:560–4.
Casadevall N, Nataf J, Viron B. Pure red-cell aplasia and anti-erythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469–75.
Everds NE, Tarrant JM. Unexpected hematologic effects of biotherapeutics in nonclinical species and in humans. Toxicol Pathol. 2013;41:280–302.
Farrell RA, Marta M, Gaeguta AJ. Development of resistance to biologic therapies with reference to IFNb. Rheumatology. 2012;51:590–9.
Ratanji KD, Derrick JP, Dearman RJ, Kimber IJ. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;11(2):99–109.
Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:501–7.
Sauerborn M, Brinks V, Jiskoot W, Schellekens H. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci. 2010;31:53–9.
Aguzzi A, O’Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9(3):237–48.
Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447(7143):453–7.
Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MF. A role for protein misfolding in immunogenicity of biopharmaceuticals. J Biol Chem. 2007;282(4):2229–36.
Gustot A, Raussens V, Dehousse M, Dumoulin M, Bryant CE, Ruysschaert JM, et al. Activation of innate immunity by lysozyme fibrils is critically dependent on cross-β sheet structure. Cell Mol Life Sci. 2013;70(16):2999–3012.
Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87(3):181–94.
Wang W, Singh SK, Li N, Toler MR, King KR, Nema S. Immunogenicity of protein aggregates–concerns and realities. Int J Pharm. 2012;431(1–2):1–11.
Engelsman J, Garidel P, Smulders R, Koll H, Smith B, Bassarab S, et al. Strategies for the assessment of protein aggregates in pharmaceutical biotech product development. Pharm Res. 2011;28:920–33.
Wiesbauer J, Prassl R, Nidetzky B. Renewal of the air-water interface as a critical system parameter of protein stability: aggregation of the human growth hormone and its prevention by surface-active compounds. Langmuir. 2013;29(49):15240–50.
Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203(1–2):1–60.
Stathopulos PB, Scholz GA, Hwang YM, Rumfeldt JA, Lepock JR, Meiering EM. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2004;13(11):3017–27.
Ruiz L, Reyes N, Aroche K, Tolosa V, Simanca V, Rogríguez T, et al. Influence of packaging material on the liquid stability of interferon-alpha2b. J Pharm Sci. 2005;8(2):207–16.
Gerhardt A, Mcgraw NR, Schwartz DK, Bee JS, Carpenter JF, Randolph TW. Protein aggregation and particle formation in prefilled glass syringes. J Pharm Sci. 2014;103(6):1601–12.
Chi EY, Weickmann J, Carpenter JF, Manning MC, Randolph TW. Heterogeneous nucleation-controlled particulate formation of recombinant human platelet-activating factor acetylhydrolase in pharmaceutical formulation. J Pharm Sci. 2005;94(2):256–74.
Akers MJ, Vasudevan V, Stickelmyer M. Formulation development of protein dosage forms. In: Nail SL, Akers MJ, editors. Development and manufacture of protein pharmaceuticals. New York: Kluwer Academic/Plenum Press; 2002. p. 47–127.
Tyagli AK, Randolph TW, Dong A, Maloney KM, Hitscherich Jr C, Carpenter JF. IgG particle formation during filling pump operation: a case study of heterogeneous nucleation on stainless steel nanoparticles. J Pharm Sci. 2009;98:94–104.
Kerwin BA, Akers MJ, Apostol I, Moore-Einsel C, Etter JE, Hess E, et al. Acute and long-term stability studies of deoxy hemoglobin and characterization of ascorbate-induced modifications. J Pharm Sci. 1999;88:79–88.
Hawe A, Friess W. Stabilization of a hydrophobic recombinant cytokine by human serum albumin. J Pharm Sci. 2007;96:2987–99.
Jones LS, Kaufmann A, Middaugh CR. Silicone oil induced aggregation of proteins. J Pharm Sci. 2005;94:918–27.
Thirumangalathu R, Krishnan S, Ricci MS, Brems DN, Randolph TW, Carpenter JF. Silicone oil- and agitation-induced aggregation of a monoclonal antibody in aqueous solution. J Pharm Sci. 2009;98:3167–81.
Carpenter JF, Randolph TW, Jiskoot W. Overlooking subvisible particles in therapeutic protein products: gaps that may compromise product quality. J Pharm Sci. 2009;98:1201–5.
Palutke M, KuKuruga D, Wolfe D, Roher A. Flow cytometric purification of Alzheimer’s disease amyloid plaque core protein using thioflavin T. Cytometry. 1987;8(5):494–9.
Mach H, Bhambhani A, Meyer BK, Burek S, Davis H, Blue JT, et al. The use of flow cytometry for the detection of subvisible particles in therapeutic protein formulations. J Pharm Sci. 2011;100(5):1671–8.
Ludwig DB, Trotter JT, Gabrielson JP, Carpenter JF, Randolph TW. Flow cytometry: a promising technique for the study of silicone oil-induced particulate formation in protein formulations. Anal Biochem. 2011;410(2):191–9.
Ostman J, Darinskas A, Zamotin V, Liutkevicius E, Lundgren E, Morozova-Roche LA. Does the cytotoxic effect of transient amyloid oligomers from common equine lysozyme in vitro imply innate amyloid toxicity? J Biol Chem. 2005;280(8):6269–75.
Lindgren M, Sörgjerd K, Hammarström P. Detection and characterization of aggregates, prefibrillar amyloidogenic oligomers, and protofibrils using fluorescence spectroscopy. Biophys J. 2005;88(6):4200–12.
Bertoncini CW, Celej MS. Small molecule fluorescent probes for the detection of amyloid self-assembly in vitro and in vivo. Curr Protein Pept Sci. 2011;12(3):205–20.
Paslawski W, Andreasen M, Nielsen SB, Lorenzen N, Thomsen K, Kaspersen JD, et al. High stability and cooperative unfolding of α-synuclein oligomers. Biochemistry. 2014;53(39):6252–63.
EMA, Committee for Medicinal Products for Human Use. EMEA/CHMP/EWP/192217/2009 Guideline on Bioanalytical Method Validation. February 2012.
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER). Draft guidance for industry: assay development for immunogenicity testing of therapeutic proteins. December 2009.
Nolan JP, Stoner SA. A trigger channel threshold artifact in nanoparticle analysis. Cytometry A. 2013;83:301–5.
van der Vlist EJ, Nolte-’t Hoen EN, Stoorvogel W, Arkesteijn GJ, Wauben MH. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc. 2012;7:1311–26.
Wen ZQ, Torraca G, Yee CY, Li G. Investigation of contaminants in protein pharmaceuticals in pre-filled syringes by multiple micro-spectroscopies. Am Pharm Rev. 2007;10:101–7.
Nishi H, Mathäs R, Fürst R, Winter G. Label-free flow cytometry analysis of subvisible aggregates in liquid IgG1 antibody formulations. J Pharm Sci. 2014;103(1):90–9.
van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12(7):1182–92.
Shapiro HM. Practical flow cytometry, 4th edition. Vienna, Austria:Wiley-Liss; 2003.
Jahn TR, Radford SE. The Yin and Yang of protein folding. FEBS J. 2005;272(23):5962–70.
Wuchner K, Büchler J, Spycher R, Dalmonte P, Volkin DB. Development of a microflow digital imaging assay to characterize protein particulates during storage of a high concentration IgG1 monoclonal antibody formulation. J Pharm Sci. 2010;99:3343–61.
Zölls S, Gregoritza M, Tantipophan R, Wiggenhom M, Winter G, Friess W, et al. How subvisible particles become invisible—relevance of the refractive index for protein particle analyses. J Pharm Sci. 2013;102:1434–46.
Fries A. Drug delivery of sensitive biopharmaceuticals with prefilled syringes. Drug Deliv Technol. 2009;9:22–7.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Elise Langdon-Neuner and Karima Benamara for editing the manuscript.
This work was supported by Baxter Innovation GmbH. C.L., M.M., T.P., T.W., P.M., P.L.T., F.S. and B.M.R. are employees of Baxter Innovation GmbH.
Authors Contribution
C. L. designed research, performed flow cytometric analysis, analyzed and interpreted data, and wrote the paper; M.M. designed research, analyzed and interpreted data, and wrote the paper; T.P. performed flow cytometric analysis, and analyzed and interpreted data, T.W. performed flow cytometric analysis of the method validation; P.M. performed, analyzed and interpreted micro flow imaging data; P.L.T. interpreted data; F.S. interpreted data; B.M.R. designed research, analyzed and interpreted data, and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 124 kb)
Rights and permissions
About this article
Cite this article
Lubich, C., Malisauskas, M., Prenninger, T. et al. A Flow-Cytometry-Based Approach to Facilitate Quantification, Size Estimation and Characterization of Sub-visible Particles in Protein Solutions. Pharm Res 32, 2863–2876 (2015). https://doi.org/10.1007/s11095-015-1669-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1669-3