Abstract
Purpose
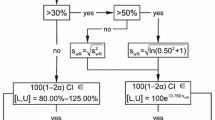
We investigated different evaluation strategies for bioequivalence trials with highly variable drugs on their resulting empirical type I error and empirical power. The classical ‘unscaled’ crossover design with average bioequivalence evaluation, the Add-on concept of the Japanese guideline, and the current ‘scaling’ approach of EMA were compared.
Methods
Simulation studies were performed based on the assumption of a single dose drug administration while changing the underlying intra-individual variability.
Results
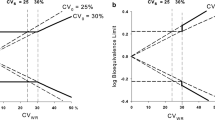
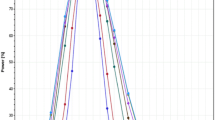
Inclusion of Add-on subjects following the Japanese concept led to slight increases of the empirical α-error (≈7.5%). For the approach of EMA we noted an unexpected tremendous increase of the rejection rate at a geometric mean ratio of 1.25. Moreover, we detected error rates slightly above the pre-set limit of 5% even at the proposed ‘scaled’ bioequivalence limits.
Conclusions
With the classical ‘unscaled’ approach and the Japanese guideline concept the goal of reduced subject numbers in bioequivalence trials of HVDs cannot be achieved. On the other hand, widening the acceptance range comes at the price that quite a number of products will be accepted bioequivalent that had not been accepted in the past. A two-stage design with control of the global α therefore seems the better alternative.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variances
- AUC:
-
Area under the curve
- BE:
-
Bioequivalence
- Cmax :
-
Maximum drug concentration
- CV:
-
Coefficient of variance
- CVANOVA :
-
Coefficient of variance calculated from the residual error in an ANOVA
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- GMR:
-
Geometric mean ratio
- HVD:
-
Highly variable drug
- k:
-
Regulatory constant of 0.760
- L:
-
Lower acceptance limit for bioequivalence
- Sw :
-
Residual error of an ANOVA calculation
- SWR :
-
Residual error of an ANOVA calculation of two Reference administrations in a partial replicate study design
- U:
-
Upper acceptance limit for bioequivalence
References
Guideline on the Investigation of Bioequivalence (CPMP/EWP/QWP/1401/98 Rev. 1/Corr, January 2010.
Hauck WW, Hauschke D, Diletti E, Bois FY, Steinijans VW, Anderson S. Choice of student's t- or Wilcoxon-based confidence intervals for assessment of average bioequivalence. J Biopharm Stat. 1997;7(1):179–89.
Schuirmann DJ. A comparison of the two one sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–80.
Note for Guidance on the Investigation of Bioavailability and Bioequivalence (CPMP/EWP/QWP/1401/98). January 2002.
Tothfalusi L, Endrenyi L, Arieta AG. Evaluation of bioequivalence for highly variable drugs with scaled average bioequivalence. Clin Pharmacokinet. 2009;48(11):725–43.
Karalis V, Symilides M, Macheras P. On the leveling-off properties of the new bioequivalence limits for highly variable drugs of the EMA guideline. Eur J Pharm Sci. 2011;44(4):497–505.
Patterson SD, Zariffa NMD, Montague TH, Howland K. Non-traditional study designs to demonstrate average bioequivalence for highly variable drug products. Eur J Clin Pharmacol. 2001;57:663–70.
Karalis V, Sylmillides M, Macheras P. Bioequivalence of highly variable drugs: a comparison of the newly proposed regulatory approaches by FDA and EMA. Pharm Res. 2012;29(4):1066–77.
Howe WG. Approximate confidence limits on the mean of X + Y where X and Y are two tabled independent variables. J Am Stat Assoc. 1974;69:789–94.
Patnaik RN, Lesko LJ, Chen ML, Williams RL. Individual bioequivalence: new concepts in the statistical assessment of bioequivalence metrics. Clin Pharmacokinet. 1997;33:1–6.
Chen ML, Lesko LJ. Individual bioequivalence revisited. Clin Pharmacokinet. 2001;40(10):701–6.
Phillips KF. Power of the two one-sided tests procedure in bioequivalence. J Pharmacokinet Biopharm. 1990;18:137–44.
Tothfalusi L, Endrenyi L, Midha KK. Scaling or wider bioequivalence limits for highly variable drugs and for the special case of C(max). Int J Clin Pharmacol Ther. 2003;41(5):217–25.
Tothfalusi L, Endrenyi L. Limits for the scaled average bioequivalence of highly variable drugs and drug products. Pharm Res. 2003;20(3):382–9.
Tothfalusi L, Endrenyi L, Midha KK, Rawson MJ, Hubbard JW. Evaluation of the bioequivalence of highly-variable drugs and drug products. Pharm Res. 2002;18(6):728–33. Comment in Pharm Res 19(3):227–8.
Haider SH, Davit B, Chen ML, Connor D, Lee LM, Li QH, et al. Bioequivalence approaches for highly variable drugs and drug products. Pharm Res. 2008;25(1):237–41.
Haider SH, Makhlouf F, Schuirmann DJ, Hyslop T, Davit B, Conner D, et al. Evaluation of a scaling approach for the bioequivalence of highly variable drugs. AAPS. 2008;10(3):450–4.
Endrenyi L, Tothfalusi L. Regulatory conditions for the determination of bioequivalence of highly variable drugs. J Pharm Sci. 2009;12(1):138–49.
Davit BM, Chen ML, Conner DP, Haidar SH, Kim S, Lee CH, et al. Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products by the US food and drug administration. AAPS J. 2012;14(4):915–24.
Guideline for Bioequivalence Studies of Generic Products, 医薬品の生物学的同等性試 験ガイドライン,発医薬品の生物学的同 等性試験ガイドライン, 発医薬品の生物学的 同等性試験ガイドライン, Ministry of Health, Labour and Welfare (MHLW). Japan; 2012.
Chow SC, Liu JP. Design and analysis of clinical trials. 2nd ed. Hoboken: Wiley-Interscience; 2004. p. 483. ISBN 0-471-24985-8.
Tothfalusi L, Endrenyi L. Sample sizes for designing bioequivalence studies for highly variable drugs. J Pharm Pharm Sci. 2012;15(1):73–84. www.cspsCanada.org.
SAS Institute Inc. SAS/STAT 9.2 SAS User’s Guide. Cary, North Carolina, USA: SAS; 2002–2008.
GraphPad Prism version 5.04 for Windows. La Jolla California USA: GraphPad Software Inc.
Wonnemann M. Different approaches for the assessment of bioequivalence of highly variable drug products—comparison of rules given in the current European, Canadian and Japanese guidelines. Master thesis, Ruprecht-Karls University of Heidelberg; 2012.
Guidance notes for applicants for consent to distribute new and changed medicines and related products, New Zealand Medicines and medical devices safety authority (MedSafe) New Zealand Regulatory Guidelines for Medicines. New Zealand; 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meinolf Wonnemann and Cornelia Frömke contributed equally to this project and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Wonnemann, M., Frömke, C. & Koch, A. Inflation of the Type I Error: Investigations on Regulatory Recommendations for Bioequivalence of Highly Variable Drugs. Pharm Res 32, 135–143 (2015). https://doi.org/10.1007/s11095-014-1450-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1450-z