ABSTRACT
Purpose
This study investigated whether haplotypes in the multidrug resistance 1 (MDR1) gene had effects on mRNA expression levels of MDR1 and cytochrome P450 (CYP) 3A4, and on the pharmacokinetics of tacrolimus in living-donor liver transplant (LDLT) patients, considering the gender difference.
Methods
Haplotype analysis of MDR1 with G2677T/A and C3435T was performed in 63 de novo Japanese LDLT patients (17 to 55 years; 44.4% women). The expression levels of MDR1 and CYP3A4 mRNAs in jejunal biopsy specimens were quantified by real-time PCR.
Results
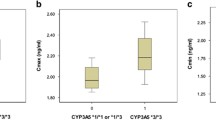
Intestinal CYP3A4 mRNA expression levels (amol/µg total RNA) showed significantly higher values in women carrying the 2677TT-3435TT haplotype (median, 10.7; range, 5.92–15.2) than those with 2677GG-3435CC (3.03; range 1.38–4.68) and 2677GT-3435CT (median, 4.31; range, 0.07–9.42) (P = 0.022), but not in men (P = 0.81). However, MDR1 haplotype did not influence mRNA expression levels of MDR1 nor the concentration/dose ratio [(ng/mL)/(mg/day)] of oral tacrolimus for the postoperative 7 days, irrespective of gender.
Conclusion
MDR1 haplotype may have a minor association with the tacrolimus pharmacokinetics after LDLT, but could be a good predictor of the inter-individual variation of intestinal expression of CYP3A4 in women.


Similar content being viewed by others
Abbreviations
- C/D:
-
Concentration/dose
- CYP:
-
Cytochrome P450
- LDLT:
-
Living-donor liver transplantation
- MDR:
-
Multidrug resistance
- Pgp:
-
P-glycoprotein
- SNP:
-
Single nucleotide polymorphism
References
F. Thiebaut, T. Tsuruo, H. Hamada, M. M. Gottesman, I. Pastan, and M. C. Willingham. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 84:7735–8 (1987). doi:10.1073/pnas.84.21.7735.
S. V. Ambudkar, S. Dey, C. A. Hrycyna, M. Ramachandra, I. Pastan, and M. M. Gottesman. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 39:361–98 (1999). doi:10.1146/annurev.pharmtox.39.1.361.
Y. Kimura, S. Y. Morita, M. Matsuo, and K. Ueda. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 98:1303–10 (2007). doi:10.1111/j.1349-7006.2007.00538.x.
S. Hoffmeyer, O. Burk, O. von Richter, H. P. Arnold, J. Brockmoller, A. Johne, I. Cascorbi, T. Gerloff, I. Roots, M. Eichelbaum, and U. Brinkmann. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 97:3473–8 (2000). doi:10.1073/pnas.050585397.
U. Brinkmannand, and M. Eichelbaum. Polymorphisms in the ABC drug transporter gene MDR1. Pharmacogenomics J. 1:59–64 (2001).
C. Marzolini, E. Paus, T. Buclin, and R. B. Kim. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 75:13–33 (2004). doi:10.1016/j.clpt.2003.09.012.
M. Goto, S. Masuda, H. Saito, S. Uemoto, T. Kiuchi, K. Tanaka, and K. Inui. C3435T polymorphism in the MDR1 gene affects the enterocyte expression level of CYP3A4 rather than Pgp in recipients of living-donor liver transplantation. Pharmacogenetics. 12:451–7 (2002). doi:10.1097/00008571-200208000-00005.
J. Lamba, S. Strom, R. Venkataramanan, K. E. Thummel, Y. S. Lin, W. Liu, C. Cheng, V. Lamba, P. B. Watkins, and E. Schuetz. MDR1 genotype is associated with hepatic cytochrome P450 3A4 basal and induction phenotype. Clin Pharmacol Ther. 79:325–38 (2006). doi:10.1016/j.clpt.2005.11.013.
I. A. Macphee, S. Fredericks, T. Tai, P. Syrris, N. D. Carter, A. Johnston, L. Goldberg, and D. W. Holt. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 74:1486–9 (2002). doi:10.1097/00007890-200212150-00002.
H. Zheng, S. Webber, A. Zeevi, E. Schuetz, J. Zhang, P. Bowman, G. Boyle, Y. Law, S. Miller, J. Lamba, and G. J. Burckart. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 3:477–83 (2003). doi:10.1034/j.1600-6143.2003.00077.x.
X. Zhang, Z. H. Liu, J. M. Zheng, Z. H. Chen, Z. Tang, J. S. Chen, and L. S. Li. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after renal transplantation. Clin Transplant. 19:638–43 (2005). doi:10.1111/j.1399-0012.2005.00370.x.
R. B. Kim, B. F. Leake, E. F. Choo, G. K. Dresser, S. V. Kubba, U. I. Schwarz, A. Taylor, H. G. Xie, J. McKinsey, S. Zhou, L. B. Lan, J. D. Schuetz, E. G. Schuetz, and G. R. Wilkinson. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 70:189–99 (2001). doi:10.1067/mcp.2001.117412.
K. Tang, S. M. Ngoi, P. C. Gwee, J. M. Chua, E. J. Lee, S. S. Chong, and C. G. Lee. Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics. 12:437–50 (2002). doi:10.1097/00008571-200208000-00004.
N. N. Salama, Z. Yang, T. Bui, and R. J. Ho. MDR1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant LLC-PK1 cells. J Pharm Sci. 95:2293–308 (2006). doi:10.1002/jps.20717.
A. R. Whitney, M. Diehn, S. J. Popper, A. A. Alizadeh, J. C. Boldrick, D. A. Relman, and P. O. Brown. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci USA. 100:1896–901 (2003). doi:10.1073/pnas.252784499.
R. Wolbold, K. Klein, O. Burk, A. K. Nussler, P. Neuhaus, M. Eichelbaum, M. Schwab, and U. M. Zanger. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 38:978–88 (2003).
T. Hashida, S. Masuda, S. Uemoto, H. Saito, K. Tanaka, and K. Inui. Pharmacokinetic and prognostic significance of intestinal MDR1 expression in recipients of living-donor liver transplantation. Clin Pharmacol Ther. 69:308–16 (2001). doi:10.1067/mcp.2001.115142.
S. Masuda, S. Uemoto, T. Hashida, Y. Inomata, K. Tanaka, and K. Inui. Effect of intestinal P-glycoprotein on daily tacrolimus trough level in a living-donor small bowel recipient. Clin Pharmacol Ther. 68:98–103 (2000). doi:10.1067/mcp.2000.107912.
M. Yasuhara, T. Hashida, M. Toraguchi, Y. Hashimoto, M. Kimura, K. Inui, R. Hori, Y. Inomata, K. Tanaka, and Y. Yamaoka. Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc. 27:1108–1110 (1995).
J. K. Lamba, Y. S. Lin, E. G. Schuetz, and K. E. Thummel. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 54:1271–1294 (2002). doi:10.1016/S0169-409X(02)00066-2.
L. Wojnowski. Genetics of the variable expression of CYP3A in humans. Ther Drug Monit. 26:192–199 (2004). doi:10.1097/00007691-200404000-00019.
T. Hirota, I. Ieiri, H. Takane, S. Maegawa, M. Hosokawa, K. Kobayashi, K. Chiba, E. Nanba, M. Oshimura, T. Sato, S. Higuchi, and K. Otsubo. Allelic expression imbalance of the human CYP3A4 gene and individual phenotypic status. Hum Mol Genet. 13:2959–2969 (2004). doi:10.1093/hmg/ddh313.
C. Kimchi-Sarfaty, J. M. Oh, I. W. Kim, Z. E. Sauna, A. M. Calcagno, S. V. Ambudkar, and M. M. Gottesman. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 315:525–528 (2007). doi:10.1126/science.1135308.
B. Goodwin, M. R. Redinbo, and S. A. Kliewer. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol. 42:1–23 (2002). doi:10.1146/annurev.pharmtox.42.111901.111051.
M. N. Jacobs, M. Dickins, and D. F. Lewis. Homology modelling of the nuclear receptors: human oestrogen receptorbeta (hERbeta), the human pregnane-X-receptor (PXR), the Ah receptor (AhR) and the constitutive androstane receptor (CAR) ligand binding domains from the human oestrogen receptor alpha (hERalpha) crystal structure, and the human peroxisome proliferator activated receptor alpha (PPARalpha) ligand binding domain from the human PPARgamma crystal structure. J Steroid Biochem Mol Biol. 84:117–132 (2003). doi:10.1016/S0960-0760(03)00021-9.
J. M. Pascussi, S. Gerbal-Chaloin, L. Drocourt, P. Maurel, and M. J. Vilarem. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta. 1619:243–253 (2003).
W. Y. Kimand, and L. Z. Benet. P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res. 21:1284–1293 (2004). doi:10.1023/B:PHAM.0000033017.52484.81.
J. Wang, A. Zeevi, K. McCurry, E. Schuetz, H. Zheng, A. Iacono, K. McDade, D. Zaldonis, S. Webber, R. M. Watanabe, and G. J. Burckart. Impact of ABCB1 (MDR1) haplotypes on tacrolimus dosing in adult lung transplant patients who are CYP3A5 *3/*3 non-expressors. Transpl Immunol. 15:235–240 (2006). doi:10.1016/j.trim.2005.08.001.
D. Anglicheau, C. Verstuyft, P. Laurent-Puig, L. Becquemont, M. H. Schlageter, B. Cassinat, P. Beaune, C. Legendre, and E. Thervet. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 14:1889–1896 (2003). doi:10.1097/01.ASN.0000073901.94759.36.
N. Tsuchiya, S. Satoh, H. Tada, Z. Li, C. Ohyama, K. Sato, T. Suzuki, T. Habuchi, and T. Kato. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 78:1182–1187 (2004). doi:10.1097/01.TP.0000137789.58694.B4.
B. Chowbay, H. Li, M. David, Y. B. Cheung, and E. J. Lee. Meta-analysis of the influence of MDR1 C3435T polymorphism on digoxin pharmacokinetics and MDR1 gene expression. Br J Clin Pharmacol. 60:159–171 (2005). doi:10.1111/j.1365-2125.2005.02392.x.
I. Cascorbi. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 112:457–473 (2006). doi:10.1016/j.pharmthera.2006.04.009.
C. Kimchi-Sarfaty, A. H. Marple, S. Shinar, A. M. Kimchi, D. Scavo, M. I. Roma, I. W. Kim, A. Jones, M. Arora, J. Gribar, D. Gurwitz, and M. M. Gottesman. Ethnicity-related polymorphisms and haplotypes in the human ABCB1 gene. Pharmacogenomics. 8:29–39 (2007). doi:10.2217/14622416.8.1.29.
D. L. Kroetz, C. Pauli-Magnus, L. M. Hodges, C. C. Huang, M. Kawamoto, S. J. Johns, D. Stryke, T. E. Ferrin, J. DeYoung, T. Taylor, E. J. Carlson, I. Herskowitz, K. M. Giacomini, and A. G. Clark. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 13:481–94 (2003). doi:10.1097/00008571-200308000-00006.
ACKNOWLEDGEMENTS
This work was supported in part by the 21st Century Center of Excellence (COE) Program “Knowledge Information Infrastructure for Genome Science”, and by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. K. Hosohata was supported as a Research Assistant by the 21st Century COE program “Knowledge Information Infrastructure for Genome Science”.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosohata, K., Masuda, S., Yonezawa, A. et al. MDR1 Haplotypes Conferring an Increased Expression of Intestinal CYP3A4 Rather than MDR1 in Female Living-Donor Liver Transplant Patients. Pharm Res 26, 1590–1595 (2009). https://doi.org/10.1007/s11095-009-9867-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9867-5