Abstract
Purpose
The purpose of this study was to increase the dissolution rate of the poorly water soluble, antifungal drug Itraconazole.
Methods
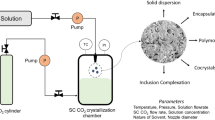
Itraconazole was successfully micronized using both the gas antisolvent (GAS) and aerosol solvent extraction systems (ASES) using Acetone as the solvent. The affects of operating conditions such as temperature, pressure and solvent choice on variables such as morphology, particle size and dissolution were investigated. The influence of temperature in the range 25 to 40°C and pressure between 90 and 190 bar were investigated.
Results
Solvent choice was found to have the largest affect on particle production, with acetone found to be the optimal solvent choice when compared with dimethyl formamide (DMF), tetrahydrofuran (THF) and dichloromethane (DCM). Itraconazole particles with an average particle size of 6.9 μm were formed at the optimal ASES processing conditions of 40°C and 190 bar. More significantly, in the first 100 minutes of dissolution 71.1% of the dense gas processed itraconazole was dissolved compared with 52.5% of Sporonox (the commercially available formulation) and 14.6% of the unprocessed material. Additional studies demonstrated that the formation of an itraconazole/PEG composite resulted in a 6-fold increase in dissolution rate in the first 100 min, to 89.8%, when compared to the unprocessed material.
Conclusions
Using ASES, microparticles of itraconazole were produced with an increased dissolution rate compared with raw material and commercially available product.











Similar content being viewed by others
References
P. J. Sinko (eds.). Martin’s Physical Pharmacy and Pharmaceutical Sciences, 2006.
R. Lobenberg, and G. L. Amidon. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur. J. Pharm. Biopharm. 50:3–12 (2000).
J.-w. Kwon, J.-h. Kim, H.-s. Wang, S.-w. Jang, and W.-t. Bae. 99-KR8542001041765, 2001.
C. German and R. Sloan. 2005–228541, 2006062848 (2006).
E. Donohoo (ed.). MIMS Online Database, 2006.
A. Engwicht, U. Girreser, and B. W. Muller. Critical properties of lactide-co-glycolide polymers for the use in microparticle preparation by the Aerosol Solvent Extraction System. Int. J. Pharm. 185:61–72 (1999).
T. J. Young, K. P. Johnston, K. Mishima, and H. Tanaka. Encapsulation of lysozyme in a biodegradable polymer by precipitation with a vapor-over-liquid antisolvent. J. Pharm. Sci. 88:640–650 (1999).
R. Ghaderi, P. Artursson, and J. Carlfors. A new method for preparing biodegradable microparticles and entrapment of hydrocortisone in dl-PLG microparticles using supercritical fluids. Eur. J. Pharm. Sci. 10:1–9 (2000).
T. Henning. Polyethylene glycols (PEGs) and the pharmaceutical industry. PharmaChem. 1:57–59 (2002).
O. I. Corrigan. Mechanisms of dissolution of fast release solid dispersions. Drug Dev. Ind. Pharm. 11:697–724 (1985).
J. L. Ford, and M. H. Rubinstein. Phase equilibria and dissolution rates of indomethacin-polyethylene glycol 6000 solid dispersions. Pharm. Acta Helv. 53:327–332 (1978).
D. Q. M. Craig. Polyethylene glycols and drug release. Drug Dev. Ind. Pharm. 16:2501–2526 (1990).
S. Verheyen, N. Blaton, R. Kinget, and G. Van den Mooter. Mechanism of increased dissolution of diazepam and temazepam from polyethylene glycol 6000 solid dispersions. Int. J. Pharm. 249:45–58 (2002).
S. Anguiano-Igea, F. J. Otero-Espinar, J. L. Vila Jato, and J. Blanco-Mendez. Clofibrate/PEG solid dispersions. The effect of PEG molecular weight and drug ratio on clofibrate release. Congr. Int. Technol. Pharm. 6th. 5:440–447 (1992).
S. Anguiano-Igea, F. J. Otero-Espinar, J. L. Vila-Jato, and J. Blanco-Mendez. The properties of solid dispersions of clofibrate in polyethylene glycols. Pharm. Acta Helv. 70:57–66 (1995).
Y. Wantanabe, H. Fujito, K. Takayama, K. Chiba, and M. Matsumoto. Consolidation and drug release characteristics of Macrogol matrix tablets containing nifedipine and increased bioavailability of nifedipine from these tablets in rabbits. Yakuzaigaku. 55:198–203 (1995).
J. Swarbrick, and J. C. Boylan (eds.). Dry granulation. Encycl. Pharm. Technol. 4:1988–1998.
J. Swarbrick, and J. C. Boylan (eds.). Wet granulation. Encycl. Pharm. Technol. 16:1988–1998.
J. Swarbrick, and J. C. Boylan (eds.). Spray drying and spray congealing of pharmaceuticals. Encycl. Pharm. Technol. 14:1988–1998.
R. O. Williams, K. P. Johnston, T. J. Young, T. L. Rogers, M. K. Barron, Z. Yu, and J. Hu. 2002–273730 2004022861 (2004).
N. R. Foster, F. Dehghani, K. M. Charoenchaitrakool, and B. Warwick. Application of dense gas techniques for the production of fine particles. Pharm. Sci. 5:105–111 (2003).
B. Subramaniam, R. A. Rajewski, and K. Snavely. Pharmaceutical processing with supercritical carbon dioxide. J. Pharm. Sci. 86:885–890 (1997).
I. Kikic, and P. Sist. Applications of supercritical fluids to pharmaceuticals: controlled drug release systems. NATO Science Series, Series E: Applied Sciences 366:291–306 (2000).
J. Jung, and M. Perrut. Particle design using supercritical fluids: Literature and patent survey. J. Supercrit. Fluids 20:179–219 (2001).
U. B. Kompella, and K. Koushik. Preparation of drug delivery systems using supercritical fluid technology. Crit. Rev. Ther. Drug Carr. Syst. 18:173–199 (2001).
M. Perrut, J. Jung, and F. Leboeuf. Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fluid processes. Part II: Preparation of composite particles. Int. J. Pharm. 288:3–10 (2005).
M. Perrut, J. Jung, and F. Leboeuf. Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fluid processes. Part I: Micronization of neat particles. Int. J. Pharm. 288:3–10 (2005).
T. Gamse, S. Schwinghammer, and R. Marr. Preparation of very fine particles using supercritical fluids. Chemie Ingenieur Technik 77:669–680 (2005).
E. Reverchon, and R. Adami. Nanomaterials and supercritical fluids. J. Supercrit. Fluids 37:1–22 (2006).
M. Bahrami, and S. Ranjbarian. Production of micro- and nano-composite particles by supercritical carbon dioxide. J. Supercrit. Fluids 40:263–283 (2007).
B. Warwick, F. Dehghani, N. R. Foster, J. R. Biffin, and H. L. Regtop. Micronization of copper indomethacin using gas antisolvent processes. Ind. Eng. Chem. Res. 41:1993–2004 (2002).
E. Reverchon, and G. Della Porta. Production of antibiotic micro- and nano-particles by supercritical antisolvent precipitation. Powder Technol. 106:23–29 (1999).
B. De Gioannis, P. Jestin, and P. Subra. Morphology and growth control of griseofulvin recrystallized by compressed carbon dioxide as antisolvent. J. Cryst. Growth 262:519–526 (2004).
S. Lee, K. Nam, S. Kim Min, W. Jun Seoung, J.-S. Park, S. Woo Jong, and S.-J. Hwang. Preparation and characterization of solid dispersions of itraconazole by using aerosol solvent extraction system for improvement in drug solubility and bioavailability. Arch. Pharm. Res. 28:866–874 (2005).
S. Lee, K. Nam, M. S. Kim, S. W. Jun, J.-S. Park, J. S. Woo, and S.-J. Hwang. Preparation and characterization of solid dispersions of itraconazole by using aerosol solvent extraction system for improvement in drug solubility and bioavailability. Arch. Pharm. Res. 28:866–874 (2005).
S.-Y. Lee, J.-K. Kim, W.-S. Kim, J.-H. Ryu, and G.-B. Lim. Study of a supercritical fluid process for the preparation of hydroxypropyl-b-cyclodextrin inclusion complexes. Hwahak Konghak 43:110–117 (2005).
A. Hassan, Y.-M. Tang, and W. Ayres James. Itraconazole formation using supercritical carbon dioxide. Drug Dev. Ind. Pharm. 30:1029–1035 (2004).
G. Verreck, A. Decorte, K. Heymans, J. Adriaensen, D. Cleeren, A. Jacobs, D. Liu, D. Tomasko, A. Arien, J. Peeters, P. Rombaut, G. Van den Mooter, and M. E. Brewster. The effect of pressurized carbon dioxide as a temporary plasticizer and foaming agent on the hot stage extrusion process and extrudate properties of solid dispersions of itraconazole with PVP-VA 64. Eur. J. Pharm. Sci. 26:349–358 (2005).
C. S. Connon, R. F. Falk, and T. W. Randolph. Role of crystallinity in retention of polymer particle morphology in the presence of compressed carbon dioxide. Macromolecules 32:1890–1896 (1999).
H. K. Chan, and D. J. W. Grant. Influence of compaction on the intrinsic dissolution rate of modified acetaminophen and adipic acid crystals. Int. J. Pharm. 57:117–124 (1989).
C. Doherty, and P. York. Mechanisms of dissolution of frusemide/PVP solid dispersions. Int. J. Pharm. 34:197–205 (1987).
S. P. Velaga, R. Berger, and J. Carlfors. Supercritical fluids crystallization of budesonide and flunisolide. Pharm. Res. 19:1564–1571 (2002).
P. Subra, and P. Jestin. Screening Design of Experiment (DOE) applied to supercritical antisolvent process. Ind. Eng. Chem. Res. 39:4178–4184 (2000).
S.-D. Yeo, and E. Kiran. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 34:287–308 (2005).
FDA, Guidance for Industry Impurities: Residual Solvents in New Veterinary Medical Products, Active Substances and Excipients U.S.D.o.H.a.H. Services, Editor, Food and Drug Administration, 2001.
H. Steckel, J. Thies, and B. W. Muller. Micronizing of steroids for pulmonary delivery by supercritical carbon dioxide. Int. J. Pharm. 152:99–110 (1997).
R. F. Falk, and T. W. Randolph. Process variable implications for residual solvent removal and polymer morphology in the formation of gentamycin-loaded poly (L-lactide) microparticles. Pharm. Res. 15:1233–1237 (1998).
E. Reverchon, G. Della Porta, and M. G. Falivene. Process parameters and morphology in amoxicillin micro and submicro particles generation by supercritical antisolvent precipitation. J. Supercrit. Fluids 17:239–248 (2000).
E. Reverchon, G. Della Porta, A. Di Trolio, and S. Pace. Supercritical antisolvent precipitation of nanoparticles of superconductor precursors. Ind. Eng. Chem. Res. 37:952–958 (1998).
A. R. Najafabadi, A. Vatanara, K. Gilani, and M. R. Tehrani. Formation of salbutamol sulphate microparticles using solution enhanced dispersion by supercritical carbon dioxide, Daru. Journal of Faculty of Pharmacy, Tehran University of Medical Sciences 13:1–5 (2005).
L. S. Tu, F. Dehghani, and N. R. Foster. Micronization and microencapsulation of pharmaceuticals using a carbon dioxide antisolvent. Powder Technol. 126:134–149 (2002).
M. C. Potter, and D. C. Wiggert. Mechanics of Fluids. Prentice-Hall, London, 1997.
A. H. Lefebvre. Atomization and Sprays. Hemisphere, New York, 1989.
L. A. Meure, B. Warwick, F. Dehghani, H. L. Regtop, and N. R. Foster. Increasing copper indomethacin solubility by coprecipitation with Poly(vinylpyrrolidone) using the aerosol solvent extraction system. Ind. Eng. Chem. Res. 43:1103–1112 (2004).
Acknowledgements
The authors wish to thank Eiffel Technologies for their financial support and for use of the Dissolution and Malvern apparatus. The authors would like to acknowledge and thank Nasim Annabi from the University of Sydney for conducting the XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrett, A.M., Dehghani, F. & Foster, N.R. Increasing the Dissolution Rate of Itraconazole Processed by Gas Antisolvent Techniques using Polyethylene Glycol as a Carrier. Pharm Res 25, 1274–1289 (2008). https://doi.org/10.1007/s11095-007-9479-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9479-x