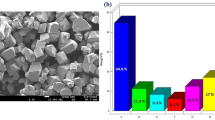
The development of drug resistant strains of pathogenic fungi despite the availability of a large number of drugs demands for the development of new, more potential drug molecules. Dendrimer-based drug molecules have been comparatively less studied upon recent advancement in this field. In the present work, a new water-soluble dendritic ligand and its copper(II), nickel(II), and cobalt(II) complexes have been synthesized. The ligand, as well as its complexes, were characterized by various physicochemical, analytical, and spectroscopic techniques. On the basis of UV-Vis spectroscopic data, tetragonal geometry was proposed for Cu(II) complex and square planar geometry was established for Co(II) and Ni(II) complexes. The antifungal activities of water-soluble compounds were evaluated using the disk diffusion method, and the minimal inhibitory concentrations (MICs) against Candida albicans ATCC 90028 were determined by the dilution method. The synthesized compounds proved to be fungicidal in comparison to fluconazole, which is fungistatic only; however, these compounds were less active than fluconazole. Hemolysis assays for one of the reported compound showed that it was nontoxic in comparison to fluconazole. Therefore, the proposed compounds can serve as promising leads for the development of new antifungal agents.






Similar content being viewed by others
References
J. Kriengkauykiat, J. I. Ito, and S. S. Dadwal, Clin. Epidemiol., 3, 175 – 191 (2011).
A. Mulu, A. Kassu, B. Anagaw, et. al., BMC Infect. Dis., 13, 82 (2013).
C. Lass-Florl, K. Griff, A. Mayr, et. al., Brit. J. Hematol., 131, 201 – 207 (2005).
M. A. Pfaller and D. J. Diekema, Clin. Microbiol. Rev., 20, 133 (2007).
B. H. Segal and T. Walsh, Am. J. Respir. Crit. Care Med., 173, 707 (2006).
M. C. Anrendrup, K. Fuursted, B. Gahrn-Hansen, et. al., J. Clin. Microbiol., 43, 4434 – 4440 (2005).
D. A. Enoch, H. A. Ludlam, and N. M. Brown, J. Med. Microbiol., 55, 809 – 817 (2006).
M. C. Arendrup, E. Dzajic, R. H. Jensen, et. al., Clin. Microbiol. Infect., 19, 343 – 353 (2003).
M. P. Wille, T. Guinmaraes, G. H. C. Furtado, and A. L. Colombo, Mem. Inst. Oswaldo Cruz, 108, 288 – 292 (2013).
M. A. Pfaller, D. J. Diekema, D. L. Gibbs, et.al., J. Clin. Microbiol., 55, 1629 – 1637 (2007).
N. C. Karyotakis and E. Anaissie, J. Curr. Opin. Inf. Dis., 7, 658 (1994).
I. A. Casalinuovo, P. Di Francesco, and E. Garaci, Eur. Rev. Med. Pharmacol. Sci. 8, 69 – 77 (2004).
C. Sheng and W. Zhang, Curr. Med. Chem., 18, 733 – 766 (2011).
D. L. Jiang and T. Aida, Nature, 388, 454 – 456 (1997).
J. W. J. Knapen, A. W. Van der Made, J. C. de Wilde, et. al., Nature, 372, 659 – 663 (1994).
S. A. Ponomarenko, E. A. Eebrov, A. Yu. Bobrovsky, et. al., Liq. Cryst., 21, 1 – 12 (1996).
P. Busson, H. Ihre, and A. Hult, J. Am. Chem. Soc., 120, 9070 – 9071 (1998).
J. F. G. A. Jansen, E. M. M. de Brabander-Van den Berg, and E. W. Meijer, Science, 266, 1226 – 1229 (1994).
C. J. Hawker and J. M. J. Frechet, J. Chem. Soc. Perkin Trans. 1, 2459 – 2469 (1992).
J. Haensler and F. C. Szoka, Bioconjugate Chem., 4, 372 – 379(1993).
C. T. Redemann and F. C. Szoka, Bioconjug. Chem., 7, 703 – 714 (1996).
E. W. Meijer, W. Paulus, and R. J. Duncan, Control. Release, 65, 133 – 148 (2000).
D. Gamovskii, A. L. Nivorozhkin, and V. I. Mimkin, Coord. Chem. Rev. 126, 1 (1993).
M. Sonmez, J. Polish, Chem. 77, 397 – 402 (2003).
M. Sonmez and M. Sekerci, Synth. React. Inorg. Met. Org. Chem., 33(10), 1747 – 1761 (2003).
M. Sonmez and M. Sekerci, Synth. React. Inorg. Met. Org. Chem., 34(3), 489 – 502 (2004).
P. A.Wayne, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, Clinical and Laboratory Standards Institute, 3rd edition (2008), p. M27-A3.
A. Ahmad, A. Khan, N. Manzoor, and L. A. Khan, Microb. Pathogen., 48, 35 – 41 (2010).
A. Chaudhary and R. V. Singh, Indian J. Chem., 43, 2529 (2004).
W. Kemp, Organic Spectroscopy, Macmillan Press Ltd., London (1975).
Ch. Krishna, C. Mohapatra, and K. C. Dash, J. Inorg. Nucl. Chem., 39, 1253 – 1258 (1977).
N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Pergamon Press, New York (1984).
M. K. Singh, R. Laskar, and A. Das, Indian. J. Chem., 41A, 2282 – 2284 (2002).
M. K. Singh, R. Laskar, and A. Das, Transit. Met. Chem., 30, 48 – 487 (2005).
A. D. Naik, S. M. Annigeri, U. B. Gangadarmath, et. al., Transit. Met. Chem., 27, 333–336 (2002).
P. P. Singh, V. P. Shukla, and R. Rivest, J. Inorg. Nucl. Chem., 37, 679 (1975).
N. Naz and M. Z. Iqbal, J. Chem. Soc. Pak., 31(3), 440 (2009).
Z. H. Chohan, H. Pervez, K. M. Khan, et. al., J. Enzyme Inhib. Med. Chem., 19(1), 85 – 90 (2004).
I. Ali, W. A. Wani, A. Khan, et. al., Microb. Pathogen., 53(2), 66 – 73 (2012).
S. Henkelman, G. Rakhorst, J. Blanton, and W. Van Oeveren, Mater. Sci. Eng. C: Biomim. Supramol. Syst., 29, 1650 – 1654 (2009).
M. M. Lubran, Clin. Lab. Sci., 19, 114 (1989).
FDA Guidance for Industry-Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients (2005); available online at: http: /www.fda.gov / downloads / drugs / guidanceComplianceRegulatoryInformation / Guidances / ucm079250.pdf
5. Acknowledgements
The authors are thankful to sophisticated analytical instrumentation facility, Punjab University Chandigarh for providing ESI-MS. Jamia Hamdard University for providing facility of NMR and UGC New Delhi for providing UGC-BSR meritorious research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, U., Bukhari, M.N., Anayutullah, S. et al. Synthesis, Characterization and Biological Evaluation of Metal Complexes with Water-Soluble Macromolecular Dendritic Ligand. Pharm Chem J 49, 868–877 (2016). https://doi.org/10.1007/s11094-016-1387-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1387-0