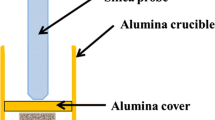
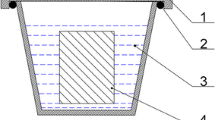
Abstract
Corrosion by molten phases leads to severe corrosion of heat exchangers in waste-to-energy plants. In addition, the presence of heavy metal chlorides in ash deposit increases degradation at low temperature due to the formation of highly corrosive molten phases. In this study, two heat exchanger materials, a low alloy steel (16Mo3) and a nickel based alloy (Inconel 625) were exposed in air to three different synthetic ashes, with various chloride contents, including ZnCl2 at isothermal temperatures of 450 and 650 °C in a muffle furnace. After the test, thickness and mass losses were evaluated on two separate samples, and metallographic cross sections of the specimens were characterized via SEM/EDX analyses. Both measurement results were in good agreement and showed that the corrosion observed on both materials was higher in the presence of zinc chloride in ash at 450 °C than in ashes without heavy metal chloride at 650 °C.













Similar content being viewed by others
References
Y. Kawahara, Corrosion Science 44, 223 (2002).
A. J. Pedersen, F. J. Frandsen, C. Riber, T. Astrup, S. N. Thomsen, K. Lundtorp and L. F. Mortensen, Energy & Fuels 23, 3475 (2009).
M. Becidan, L. Sørum, F. Frandsen and A. J. Pedersen, Fuel 88, 595 (2009).
T. Talonen, Chemical equilibria of heavy metals in waste incineration: comparison of thermodynamic databases. Lic. Thesis. (Åbo Akademi University, Finland, 2008).
Y. L. Zhang and E. Kasai, ISIJ International 44, 1457 (2004).
M. Bøjer, P. A. Jensen, F. Frandsen, K. Dam-Johansen, O. H. Madsen and K. Lundtorp, Fuel Processing Technology 89, 528 (2008).
S. Osada, D. Kuchar and H. Matsuda, Journal of Material Cycles and Waste Management 11, 367 (2009).
C. Chan, C. Q. Jia, J. W. Graydon and D. W. Kirk, Journal of Hazardous Materials 50, 1 (1996).
M. Spiegel, Materials and Corrosion 50, 373 (1999).
Y. S. Li, Y. Niu and W. T. Wu, Materials Science and Engineering: A 345, 64 (2003).
E. Schaal, N. David, P. J. Panteix, C. Rapin, J. M. Brossard and F. Maad, Oxidation of Metals 84, 307 (2015).
H. J. Grabke, Materials at High Temperatures 11, 23 (1993).
J. Lehmusto, P. Yrjas, B. J. Skrifvars and M. Hupa, Fuel Processing Technology 104, 253 (2012).
J. Pettersson, N. Folkeson, L. G. Johansson and J. E. Svensson, Oxidation of metals 76, 93 (2011).
D. Bankiewicz, P. Yrjas, D. Lindberg and M. Hupa, Corrosion Science 66, 225 (2013).
T. Jonsson, N. Folkeson, J. E. Svensson, L. G. Johansson and M. Halvarsson, Corrosion Science 53, 2233 (2011).
T. Varis, D. Bankiewicz, P. Yrjas, M. Oksa, T. Suhonen, S. Tuurna, K. Ruusuvuori and S. Holmström, Surface & Coatings Technology 265, 235 (2015).
A. Ruh and M. Spiegel, Corrosion Science 48, 679 (2006).
Y. S. Li, M. Spiegel and S. Shimada, Materials Chemistry and Physics 93, 217 (2005).
J. Lehmusto, B. J. Skrifvars, P. Yrjas and M. Hupa, Corrosion Science 53, 3315 (2011).
S. Enestam, D. Bankiewicz, J. Tuiremo, K. Mäkelä and M. Hupa, Fuel 104, 294 (2013).
C. W. Bale, P. Chartrand, S. A. Degterov, G. Eriksson, K. Hack, R. B. Mahfoud, J. Melancon, A. A. Pelton and S. Petersen, Calphad 26, 189 (2002).
ISO/DIS 17248, AFNOR, (2014)
N. P. Lushnaya, N. N. Evseeva and I. P. Vereshchetina, Russian Journal of Inorganic Chemistry 1, 35 (1956).
M. Sánchez Pastén and M. Spiegel, Materials and Corrosion 57, 192 (2006).
D. Bankiewicz, S. Enestam, P. Yrjas and M. Hupa, Fuel Processing Technology 105, 89 (2013).
A. Roine. HSC Chemistry Version 4.1. (Outokumpu Research Organization, Pori, Finland, 1999).
Acknowledgments
This work has been supported by the French National Research Agency with project ANR SCAPAC 11-RMNP-0016 in partnership with, AIR LIQUIDE, SEDIS and CIRIMAT/ENSIACET. The authors thank L. ARANDA of Institut Jean Lamour, Nancy (France) for carrying out TMA and DTA analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaal, E., David, N., Panteix, P.J. et al. Effect of Zinc Chloride in Ash in Oxidation Kinetics of Ni-Based and Fe-Based Alloys. Oxid Met 85, 547–563 (2016). https://doi.org/10.1007/s11085-016-9612-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-016-9612-5