Abstract
Formamide (NH2CHO) has been irradiated in condensed phase at 273 K by 11B-boron beams in the presence of powdered meteorites of the chondrite and stony-iron types. Relative to the controls (no radiation or no catalysis), a variegate panel of compounds was observed, including purine and pyrimidine nucleobases (uracil, cytosine, adenine, and guanine), nucleobase analogues, heterocycles, and carboxylic acids involved in metabolic pathways. The presence of amino imidazole carbonitrile (AICN), 4,6-diamino purine (4,6-DAP) and 2,4-diamino pyrimidine (2,4-DAPy) among the observed products suggests the occurrence of an unified mechanism based on the generation of radical cyanide species (•CN). These observations contribute to outline plausible prebiotic scenarios involving 11B-boron as energy source.
Similar content being viewed by others
Introduction
The interstellar abundance of boron is of great interest for the analysis of chemical evolution in our Galaxy (Jura et al. 1996). Two general mechanisms can account for the formation of boron following the Big Bang, namely: a) the “spallation reaction”, consisting in the collision between high energy Galactic Cosmic Rays (GCR) and heavier elements (such as carbon and oxygen) (Reeves et al. 1970), or b) the reaction of low energy GCR with interstellar hydrogen and helium in pre-stellar objects (Federman et al. 1995). The value of the isotopic 11B/10B ratio observed on Earth (c.a. 4.0) suggests the occurrence of the second mechanism in the Solar System, confirming the prevalence of 11B-boron in the pre-Solar Nebula. A similar isotopic ratio was observed in chondrules of meteorites of the chondrite family, such as Allende, Orgueil, Semarkona and Hedjaz (Chaussidon and Robert 1995). The elemental composition of Orgueil represents the last chemically fractionated sample relative to Sun; Allende is rich in supernovae condensates, while the deuterium enrichment in Semarkona is representative of the transformations taking place in the pristine Solar Nebula. Moreover, boron is present in the cosmic rays near the Earth (11B/10B ratio c.a 2.6, beam flux of 1.5 10−3ions/cm2/s), even though it is presumably absent in the source regions (Durgaprasad 1971). Thus, 11B-boron may have played a significant role in prebiotic processes occurring in the pristine Solar Nebula.
Formamide (NH2CHO), the simplest amide in nature, is becoming one of the most intensively studied chemical precursor for the prebiotic syntheses of compounds necessary for the origin of life (Saladino et al. 2012a; Saladino et al. 2012b; Saitta and Saija 2014; Ferus et al. 2015). Kiloparsecs-wide molecular clouds of NH2CHO were detected in the galactic habitable zone (Adande et al. 2013) and in several representative pre-stellar objects (Halfen et al. 2011). Evidence for the catalytic properties of meteorites in the prebiotic chemistry of NH2CHO was recently provided by thermal processes in condensed phase at 333 K and 413 K (Saladino et al. 2011; Saladino et al. 2013a), or at the most extreme temperature conditions modeling impact events in Earth’s atmosphere or surface (c.a. 5000 K) (Ferus et al. 2014). Recently, we reported that NH2CHO yields an extremely rich, variegate and biologically relevant panel of compounds, when irradiated by high-energy proton beams (170 MeV, 243 K) in the presence of powdered meteorites, mimicking the effect of cosmic ion irradiation (Saladino et al. 2015). As a continuation of this study, here we describe the first evidence on the role of 11B-boron irradiation of meteorites and NH2CHO in the origin of organics of biological relevance, such as nucleobases and carboxylic acids.
Materials and Methods
Formamide (Fluka, > 99 %) was used without further purification. Dhofar 959 was obtained from Cometshopnew, CastroValley, California, USA; NWA 1465 and NWA 4482 from Sahara-nayzak, Asnieres sur Seine, France; Gold Basin from AZ meteorites, Tombstone, Arizona, USA. An original sample of Chelyabinsk came from the collection of prof. A. Rozanov. Gas-chromatography mass-spectrometry (GC-MS) analyses were performed with a LC/GG MS Combo Agilent and Shimadzu GC-MS QP5050A with a Variant CP8944 column (WCOT fused silica, film thickness 0.25 μm, stationary phase VF-5 ms, Øί 0.25 mm, lenght 30 m). Dust samples of the appropriate meteorite (approximately 100 mg) were extracted by a two-steps procedure to remove organics as previously reported (Saladino et al. 2011). Briefly, the sample was treated with NaOH (0.1 N, 1.0 mL) and CHCl3-CH3OH (ratio v/v 2:1, 5.0 mL), followed by a second extraction step with sulphuric acid (0.1 N, 1.0 mL) and CHCl3-CH3OH (ratio v/v 2:1, 5.0 mL). Anyway, the amount of compounds obtained after irradiation was several orders of magnitude higher than reported endogenous organics in original meteorite samples. The powder was recovered by centrifugation (6000 rpm, 10 min) and the supernatant phase was decanted. NH2CHO (2.5 mL) and catalytic amount of the meteorite powder (1.0 % in weight; similar grain size distribution of particles in the range of 0–125 μm) were irradiated in a closed (not in vacuum) Eppendorf at 273 K with a 33 MeV per nucleon (33 MeV/n) 11B-boron beam (beam flux of 3.6 105 ions/cm2/s) generated by Cyclotron facility at the Joint International Nuclear Institute (JINR; Dubna, Russia) for 3 min. The averaged linear energy transfer (LET) was about 57.3 keV/μm and the calculated absorbed dose was 6.0 Gy. At the end of the irradiation the mineral was recovered by centrifugation (6000 rpm, 10 min, Haereus Biofuge), and the excess NH2CHO removed by distillation (40 °C, 4 × 10−4 bar). The crude product was analyzed by gas chromatography and mass spectrometry (GC-MS) after treatment with N,N-bis-trimethylsilyl trifluoroacetamide in pyridine (620 μL) at 60 °C for 4 h in the presence of betulinol (CAS number 473–98-3) as internal standard (0.2 mg). Mass spectrometry was performed by the following program: injection temperature 280 °C, detector temperature 280 °C, gradient 100 °C × 2 min, 10 °C/min for 60 min. Two strategies were applied for the identification of the structure of the products. First, the spectra were compared with commercially available electron mass spectrum libraries such as NIST (Fison, Manchester, UK). Second, GC-MS analysis was repeated with standard compounds. All products have been recognized upon a similarity index (S.I.) greater than 98 % compared to the reference standards.
Results
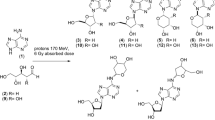
NH2CHO was irradiated with 11B-boron in the presence of catalytic amount of chondrites (Dhofar 959, Gold Basin, Chelyabinsk, and NWA 1465) and stony-iron (NWA 4482) meteorites (composition, historical and terrestrial provenience, and cosmo-origin of meteorites are in SI # 1). In particular, neat formamide (2.5 mL) mixed with the appropriate meteorite powder (1.0 % in weight) was irradiated at 273 K with a 33 MeV per nucleon (33 MeV/n) 11B beam for 3 min (Fig. 1). The averaged linear energy transfer (LET) was about 57.3 keV/μm and the calculated absorbed dose was 6.0 Gy. The values of temperature and dose were selected on the basis of what actually experienced by samples during the abiotic phosphorylation and peptide bond formation in conditions of space flight on the Biosputnik “BION-11” satellite (Kuzicheva and Simakov 1999; Kuzicheva and Gontareva 1999), and subsequently used in ground-based experiments to model chemical transformations on asteroid surfaces (Simakov 2008). The meteorite powder was previously treated to remove possible traces of organics in the original sample (observed only in the ppb range) (Cooper et al. 2011; Callahan et al. 2011). The products were analyzed by gas-chromatography mass-spectrometry (GC-MS) after formation of the corresponding trimethylsilyl ethers (TMS). The analysis was limited to products ≥1 ng/mL and the yield calculated as mg (or μg) of product per mL of starting NH2CHO. The irradiation of NH2CHO without addition of meteorite powder afforded few products in amounts lower than 0.05 μg/mL, including N,N-diethyl formamide, N,N-diethyl acetamide, 3-hydroxy pyridine, and pyruvic acid, in addition to unidentified peaks.
The treatment of NH2CHO with meteorite powder in the absence of 11B-boron irradiation did not afford products in appreciable yield, even upon prolonged reaction times (24 h). Instead, purine and pyrimidine nucleobases, some of their isomers and analogues, heterocycles, carboxylic acids, and intermediates required for the formation of biomolecules, were synthesized in the presence of meteorites (Fig. 2). Table 1 shows the most abundant products (m/z values and peak abundances are in SI # 2). Selected chromatograms and original m/z fragmentation spectra are in SI # 3. The complete set of nucleobases of RNA [uracil (1), cytosine (2), adenine (3) and guanine (4)] was obtained in different yield and selectivity depending on the meteorite used in the irradiation (Table 1). Among the chondrite meteorites, the overall nucleobases abundances were higher with Chelyabinsk and Dhofar 959 compared to Gold Basin and NWA1465. A differential distribution of indigenous nucleobases in chondrites was previously observed during extraction experiments (Burton et al. 2012). Also in this analysis, Chelyabinsk and Dhofar 959 were more active than stony-iron NWA 4482. Nucleobases were isolated in the range of 0.11 (NWA 1465, only guanine detected) to 3.21 (Chelyabinsk, complete set of RNA nucleobases) μg/mL (total amount of nucleobases), respectively.
Along with nucleobases, related analogues and isomers, including isocytosine (5), hypoxanthine (6), 2,6-diamino purine (2-DAP) (7), 2,4-diamino pyrimidine (2,4-DAPy) (8), 4,6-dihydroxy pyrimidine (4,6-DHPy) (9), and orotic acid (10), were obtained in appreciable yield. Traces of 4-amino imidazole carbonitrile (4-AICA) were detected in the case of Chelyabinsk. The biological relevance of compounds 5–6, 9 and 10, in the context of the origin of life and of chemiomimesis has been previously reviewed (Saladino et al. 2008; Saladino et al. 2013b; Hirao et al. 2012). 2-DAP (7), considered as possible extraterrestrial marker since not typically found in terrestrial biochemistry (Callahan et al. 2011), 2,4-DAPy (8) and 4-AICA (11) are key intermediates in the unified mechanism of nucleobase formation based on the multi-step addition of the cyanide radical (•CN) on NH2CHO (Ferus et al. 2014). In this process, meteorites can tune the selectivity of the condensation (Ferus et al. 2012). 4-AICA (11) is the common intermediate for adenine and guanine, while 2,4-DAPy (8) is that of cytosine and uracil (in this reaction pathway, 2-DAP (7) follows 4-AICA in the formation of guanine).
Carboxylic acids, with increasing levels of structural complexity have been identified in meteorites (Pizzarello et al. 2006). The irradiation of NH2CHO with 11B-boron and meteorites afforded pyruvic (12), lactic (13), maleic (14), oxaloacetic (15), citric (16), and parabanic (17) acids, whose yield was higher than that reported for indigenous organics in carbonaceous chondrites (Fig. 2, Table 1) (Cooper et al. 2011). In terms of biological relevance, compounds 12, 15, and 16 are intermediates of the reversed version of the citric acid cycle (Kreb cycle), one of the most ancient process in metabolism (Nelson and Cox 2000), while 13 is involved in the Cori cycle and gluconeogenesis (McArdle et al. 2010). Carboxylic acids were isolated in the range of 0.37 (Dhofar 959, only cyctric acid) to 1.11 (NWA 1465, pyruvic, oxaloacetic, citric and parabanic acids) μg/mL, respectively (total amount of carboxylic acids). As reported by Eschenmoser, the synthesis of carboxylic acids may be explained by the formation of diamino malonitrile DAMN (Eschenmoser 2007), through a process that in space condition proceeds by a radical mechanism involving nitrogen and carbon-centered species (Meinert et al. 2010). Since DAMN is also the key intermediate for the formation of nucleobases (Ferus et al. 2014), a general and unified mechanism for the origin of both nucleobases and carboxylic acids, based on the cyanide radical (•CN) formation by NH2CHO degradation, can be reasonably hypothesized.
Conclusions
11B-boron irradiation of NH2CHO and meteorites afford to produce nucleobases and carboxylic acids in appreciable amounts. A unified mechanism based on the addition of cyanide radical (•CN) on NH2CHO can account for the formation of both families of compounds. As a general trend, chondrite meteorites were more reactive than stony-iron NWA 4482. In comparison to previously reported data on the irradiation of NH2CHO and meteorites with high-energy (170 MeV) protons at the same dose (6.0 Gy) (Saladino et al. 2015), a lower variety of products was detected, nucleosides and amino acids being not observed in the reaction mixtures. Probably, the larger panel of products observed during proton irradiation relative to boron may be in part due to the different penetration depth of the ions. On the other hand, Chelyabinsk and Dhofar 959 produced higher amounts of nucleobases upon 11B-boron irradiation than after proton irradiation, probably due to the higher linear energy transfer (LET) value (that is: 53.7 keV for 11B boron versus 0.57 keV for protons). Taken together, these data further suggest that high energy particles in CR can in general effectively and non-fastidiously act as efficient energy source for the meteorites-catalysed prebiotic synthesis of organics instrumental in origin of life processes.
References
Adande GR, Woolf NJ, Ziurys LM (2013) Observations of interstellar formamide: availability of a prebiotic precursor in the galactic habitable zone. Astrobiology 13(5):439–453
Burton AS, Stern JC, Elsila JE, Glavin DP, Dworkin J-P (2012) Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem Soc Rev 41(16):5459–5472
Callahan MP, Karen E, Smith H, Cleaves J, Ruzicka J, Sterna JC, Glavin DP, House C H, Dworkin JP (2011) Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci U S A 108(34):13995–13998
Chaussidon M, Robert F (1995) Nucleosynthesis of 11B-rich boron in the pre-solar cloud recorded in meteoritic chondrules. Nature 374:337–339
Cooper G, Reed C, Nguyen D, Carter M, Wang Y (2011) Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc Natl Acad Sci U S A 108(34):14015–14020
Durgaprasad N (1971) The relative abundances of the isotopes of lithium, beryllium and boron in the primary cosmic radiation. astrophys. Space Science 12:98–103
Eschenmoser A (2007) On a hypothetical generational relationship between HCN and constituents of the reductive citric acid cycle. Chem Biodivers 4(4):554–573
Federman SR, Lambert DL, Cardelli JA, Sheffer Y (1995) The boron isotope ratio in the interstellar medium. Nature 381:764–766
Ferus M, Civis S, Mladek A, Sponer J, Juha L, Sponer J-E (2012) On the road from formamide ices to nucleobases: IR spectroscopic observation of a direct reaction between cyano radicals and formamide in a high-energy impact event. J Am Chem Soc 134:20788–20796
Ferus M, Nesvorný D, Sponer J, Kubelík P, Michalčíková R, Shestivská V, Sponer JE, Civiš S (2014) High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc Natl Acad Sci U S A 112:657–662
Ferus M, Knížek A, Civiš S (2015) Meteorite-catalyzed synthesis of nucleosides and other prebiotic compounds. Proc Natl Acad Sci U S A 112:7109–7110
Halfen DT, Ilyushin V, Ziurys LM (2011) Formation of peptide bonds in space: a comprehensive study of formamide and acetamide in SgrB2(N). Astrophys J 743:60–72
Hirao I, Kimoto M, Yamashige R (2012) Natural versus artificial creation of base pairs in DNA: origin of nucleobases from the perspectives of unnatural base pair studies. Acc Chem Res 45(12):2055–2065
Jura M, Meyer DM, Hawkins I, Cardelli JA (1996) The interstellar boron abundance toward Orion. Astrophysics 456:598–601
Kuzicheva EA, Simakov MB (1999) Abiogenic synthesis of nucleotides in conditions of space flight of the biosputnik “BION-11”. Adv Space Res 23(2):387–391
Kuzicheva EA, Gontareva NB (1999) The possibility of nucleotide abiogenic synthesis in conditions of “kosmos-2044” satellite space flight. Adv Space Res 23(2):393–396
McArdle W-D, Katch F-I, Katch V-L. (2010). Exercise physiology: Energy, nutrition, and human performance. Wolters Kluwer/Lippincott Williams & Wilkins Health, Philadelphia. ISBN 978–0-683-05731-7. ISBN 0–683-05731-6.
Meinert C, Filippi J-J, Nahon L, Hoffmann S-V, d’Hendecourt L, de Marcellus P, Bredehöft J-H, Wolfram H-P. Thiemann and Meierhenrich UJ. (2010) Photochirogenesis: photochemical models on the origin of biomolecular homochirality. Symmetry 2(2):1055–1080.
Nelson DL, Cox MM (2000) In lehninger principles of biochemistry. 3rd edn. Worth publishers. N Y 16:567–597
Pizzarello S., Cooper G. W., Flynn G. J. (2006) in Meteorites and the Early Solar System II. Univ Arizona Press, Tucson, pp 625–651.
Reeves H, Fowler WA, Hoyle F (1970) Galactic Cosmic Ray origin of Li, Be and B in stars. Nature 226:727–729
Saitta AM, Saija F (2014) Miller experiments in atomistic computer simulations. Proc Natl Acad Sci U S A 111(38):13768–13773
Saladino R, Neri V, Crestini C, Costanzo G, Graciotti M, Di Mauro E (2008) Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals. J Am Chem Soc 130(46):15512–15518
Saladino R, Crestini C, Cossetti C, Di Mauro E, Deamer D (2011) Catalytic effects of Murchison Material: Prebiotic Synthesis and Degradation of RNA Precursors. Orig Life Evol Biosph 41(5):437–451
Saladino R, Botta G, Pino S, Costanzo G, Di Mauro E (2012a) Genetics first or metabolism first? the formamide clue. Chem Soc Rev 41:5526–5565
Saladino R, Crestini C, Pino S, Costanzo G, Di Mauro E (2012b) Formamide and the origin of life. Phys Life Rev 9:84–104
Saladino R, Botta G, Pino S, Costanzo G, Di Mauro E (2013a) Materials for the onset. A story of necessity and chance. Front Biosci 18(4):1275–1289
Saladino R, Botta G, Delfino M, Di Mauro E (2013b) Meteorites as catalysts for prebiotic chemistry. Chemistry: A European J 19:16916–16922
Saladino R, Carota E, Botta G, Kapralov M, Timoshenko GN, Rozanov A, Krasavin E, Di Mauro E (2015) Meteorite-catalyzed synthesis of nucleosides and other prebiotic compounds under proton irradiation2. Proc Natl Acad Sci U S A 112:2746–2755
Simakov MB (2008) Asteroids and origin of life-two steps of chemical evolution on the surface of these objects. Earth Planets Space 60:75–82
Acknowledgments
This Work Is Supported by the Italian Space Agency (ASI) Project “Esobiologia e Ambienti Estremi: Dalla Chimica Delle Molecola Alla Biologia Degli Estremofili” Number 2014–026-R.0 (CUP: F 92I14000030005). this Work Was also Supported by COST Action TD 1308
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Saladino, R., Carota, E., Botta, G. et al. First Evidence on the Role of Heavy Ion Irradiation of Meteorites and Formamide in the Origin of Biomolecules. Orig Life Evol Biosph 46, 515–521 (2016). https://doi.org/10.1007/s11084-016-9495-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-016-9495-0