Abstract
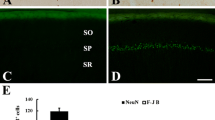
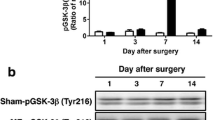
Glycogen synthase kinase 3β (GSK-3β) is a key downstream protein in the PI3K/Akt pathway. Phosphorylation of serine 9 of GSK-3β (GSK-3β activity inhibition) promotes cell survival. In this study, we examined changes in expressions of GSK-3β and phosphorylation of GSK-3β (p-GSK-3β) in the gerbil hippocampal CA1 area after 5 min of transient cerebral ischemia. GSK-3β immunoreactivity in the CA1 area was increased in pyramidal cells at 6 h after ischemia–reperfusion. It was decreased in CA1 pyramidal cells from 12 h after ischemia–reperfusion, and hardly detected in the CA1 pyramidal cells at 5 days after ischemia–reperfusion. p-GSK-3β immunoreactivity was slightly decreased in CA1 pyramidal cells at 6 and 12 h after ischemia–reperfusion. It was significantly increased in these cells at 1 and 2 days after ischemia–reperfusion. Five days after ischemia–reperfusion, p-GSK-3β immunoreactivity was hardly found in CA1 pyramidal cells. However, p-GSK-3β immunoreactivity was strongly expressed in astrocytes primarily distributed in strata oriens and radiatum. In conclusion, GSK-3β and p-GSK-3β were significantly changed in pyramidal cells and/or astrocytes in the gerbil hippocampal CA1 area following 5 min of transient cerebral ischemia. This finding indicates that GSK-3β and p-GSK-3β are closely related to delayed neuronal death.






Similar content being viewed by others
References
Lee CH, Ahn JH, Won MH (2015) New expression of 5-HT1A receptor in astrocytes in the gerbil hippocampal CA1 region following transient global cerebral ischemia. Neurol Sci 36:383–389
Lee JC, Chen BH, Cho JH et al (2015) Changes in the expression of DNA-binding/differentiation protein inhibitors in neurons and glial cells of the gerbil hippocampus following transient global cerebral ischemia. Mol Med Rep 11:2477–2485
Park OK, Lee CH, Hwang IK et al (2010) Effects of repeated restraint stress on platelet endothelial cell adhesion molecule-1 immunoreactivity and protein levels in the gerbil hippocampus after transient cerebral ischemia. Anat Cell Biol 43:54–63
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–397
Radak D, Katsiki N, Resanovic I et al (2016) Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol
Wang JK, Yu LN, Zhang FJ et al (2010) Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res 1357:142–151
Abe E, Fujiki M, Nagai Y et al (2010) The phosphatidylinositol-3 kinase/Akt pathway mediates geranylgeranylacetone-induced neuroprotection against cerebral infarction in rats. Brain Res 1330:151–157
Xu X, Chua CC, Gao J et al (2008) Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res 1227:12–18
Pap M, Cooper GM (1998) Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 273:19929–19932
Morioka M, Kawano T, Yano S et al (2006) Hyperphosphorylation at serine 199/202 of tau factor in the gerbil hippocampus after transient forebrain ischemia. Biochem Biophys Res Commun 347:273–278
Venna VR, Benashski SE, Chauhan A et al (2015) Inhibition of glycogen synthase kinase-3β enhances cognitive recovery after stroke: the role of TAK1. Learn Mem 22:336–343
Xiong T, Tang J, Zhao J et al (2012) Involvement of the Akt/GSK-3β/CRMP-2 pathway in axonal injury after hypoxic–ischemic brain damage in neonatal rat. Neuroscience 216:123–132
Kim DH, Lee HE, Kwon KJ et al (2015) Early immature neuronal death initiates cerebral ischemia-induced neurogenesis in the dentate gyrus. Neuroscience 284:42–54
Endo H, Nito C, Kamada H et al (2006) Activation of the Akt/GSK3β signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab 26:1479–1489
Radenovic L, Selakovic V, Olivan S et al (2014) Neuroprotective efficiency of tetanus toxin C fragment in model of global cerebral ischemia in Mongolian gerbils. Brain Res Bull 101:37–44
Shcherbak NS, Galagudza MM, Ovchinnikov DA et al (2013) Activity of succinate dehydrogenase in the neocortex and hippocampus of Mongolian gerbils with ischemic and reperfusion brain injury. Bull Exp Biol Med 155:14–17
Zhao XY, Wu CF, Yang J et al (2015) Effect of arginine vasopressin on the cortex edema in the ischemic stroke of Mongolian gerbils. Neuropeptides 51:55–62
Ahn JH, Choi JH, Park JH et al (2016) Long-term exercise improves memory deficits via restoration of myelin and microvessel damage, and enhancement of neurogenesis in the aged gerbil hippocampus after ischemic stroke. Neurorehabil Neural Repair 30:894–905
Yan BC, Park JH, Ahn JH et al (2012) Comparison of the immunoreactivity of Trx2/Prx3 redox system in the hippocampal CA1 region between the young and adult gerbil induced by transient cerebral ischemia. Neurochem Res 37:1019–1030
Chen BH, Park JH, Cho JH et al. (2016) Tanshinone I enhances neurogenesis in the mouse hippocampal dentate gyrus via increasing Wnt-3, phosphorylated glycogen synthase kinase-3beta and beta-catenin immunoreactivities. Neurochem Res 41:1958–1968
Seo JY, Yan BC, Park JH et al (2013) Comparison of the immunoreactivities of NMDA receptors between the young and adult hippocampal CA1 region induced by experimentally transient cerebral ischemia. J Neurol Sci 325:108–114
Yan BC, Kim SK, Park JH et al (2012) Comparison of inflammatory cytokines changes in the hippocampal CA1 region between the young and adult gerbil after transient cerebral ischemia. Brain Res 1461:64–75
Ko I-G, Shin M-S, Kim B-K et al (2009) Tadalafil improves short-term memory by suppressing ischemia-induced apoptosis of hippocampal neuronal cells in gerbils. Pharmacol Biochem Behav 91:629–635
Lee CH, Park JH, Cho JH et al (2014) Changes and expressions of Redd1 in neurons and glial cells in the gerbil hippocampus proper following transient global cerebral ischemia. J Neurol Sci 344:43–50
Peineau S, Bradley C, Taghibiglou C et al (2008) The role of GSK-3 in synaptic plasticity. Br J Pharmacol 153:S428–S437
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116:1175–1186
Kim WY, Snider WD (2011) Functions of GSK-3 signaling in development of the nervous system. Front Mol Neurosci 4:44
Kim WY, Zhou FQ, Zhou J et al (2006) Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52:981–996
Bhat RV, Shanley J, Correll MP et al (2000) Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci USA 97:11074–11079
Grimes CA, Jope RS (2001) The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 65:391–426
Linseman DA, Butts BD, Precht TA et al (2004) Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 24:9993–10002
Rehni AK, Singh N (2007) Role of phosphoinositide 3-kinase in ischemic postconditioning-induced attenuation of cerebral ischemia-evoked behavioral deficits in mice. Pharmacol Rep 59:192–198.
Zheng Y, Hou J, Liu J et al (2014) Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J Pharmacol Sci 124:354–364
Xing XS, Liu F, He ZY (2015) Akt regulates beta-catenin in a rat model of focal cerebral ischemia-reperfusion injury. Mol Med Rep 11:3122–3128
Liu H, Ou S, Xiao X et al (2015) Diabetes worsens ischemia–reperfusion brain injury in rats through GSK-3beta. Am J Med Sci 350:204–211
Zhao S, Fu J, Liu X et al (2012) Activation of Akt/GSK-3beta/beta-catenin signaling pathway is involved in survival of neurons after traumatic brain injury in rats. Neurol Res 34:400–407
Ma Y, Li Y, Zhang C et al (2014) Neuroprotective effect of 4-methylcyclopentadecanone on focal cerebral ischemia/reperfusion injury in rats. J Pharmacol Sci 125:320–328
Collino M, Thiemermann C, Mastrocola R et al (2008) Treatment with the glycogen synthase kinase-3beta inhibitor, TDZD-8, affects transient cerebral ischemia/reperfusion injury in the rat hippocampus. Shock 30:299–307
Perez-Alvarez MJ, Mateos L, Alonso A et al (2015) Estradiol and progesterone administration after pMCAO stimulates the neurological recovery and reduces the detrimental effect of ischemia mainly in hippocampus. Mol Neurobiol 52:1690–1703
Kim AH, Khursigara G, Sun X et al (2001) Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 21:893–901
Brown AM, Ransom BR (2007) Astrocyte glycogen and brain energy metabolism. Glia 55:1263–1271
Yoo DY, Lee KY, Park JH et al. (2016) Glucose metabolism and neurogenesis in the gerbil hippocampus after transient forebrain ischemia. Neural Regen Res 11:1254
Acknowledgements
This research was supported by the Bio-Synergy Research Project (NRF-2015M3A9C4076322) of the Ministry of Science, ICT and Future Planning through the National Research Foundation, and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science, ICT and Future Planning (NRF-2014R1A1A3051721).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Bai Hui Chen and Ji Hyeon Ahn they have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, B.H., Ahn, J.H., Park, J.H. et al. Transient Cerebral Ischemia Alters GSK-3β and p-GSK-3β Immunoreactivity in Pyramidal Neurons and Induces p-GSK-3β Expression in Astrocytes in the Gerbil Hippocampal CA1 Area. Neurochem Res 42, 2305–2313 (2017). https://doi.org/10.1007/s11064-017-2245-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2245-5