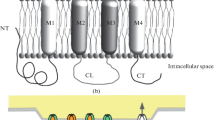
Abstract
Astrocytes in the mammalian central nervous system are interconnected by gap junctions made from connexins of the subtypes Cx30 and Cx43. These proteins may exist as hemichannels in the plasma membrane in the absence of a ‘docked’ counterpart on the neighboring cell. A variety of stimuli are reported to open the hemichannels and thereby create a permeation pathway through the plasma membrane. Cx30 and Cx43 have, in their hemichannel configuration, been proposed to act as ion channels and membrane pathways for different molecules, such as fluorescent dyes, ATP, prostaglandins, and glutamate. Published studies about astrocyte hemichannel behavior, however, have been highly variable and/or contradictory. The field of connexin hemichannel research has been complicated by great variability in the experimental preparations employed, a lack of highly specific pharmacological inhibitors and by confounding changes associated with genetically modified animal models. This review attempts to critically assess the gating, inhibition and permeability of astrocytic connexin hemichannels and proposes that connexins in their hemichannel configuration act as gated pores with isoform-specific permeant selectivity. We expect that some, or all, of the controversies discussed here will be resolved by future research and sincerely hope that this review serves to motivate such clarifying investigations.





Similar content being viewed by others
References
Harris AL (2007) Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94:120–143
Harris AL, Locke D (2009) Permeability of connexin channels. In: Harris, AL, Locke, D (eds), Connexins a guide, Humana Press, New York, pp 165–206
Nielsen MS, Nygaard AL, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH (2012) Gap junctions. Compr Physiol 2:1981–2035
Sohl G, Willecke K (2003) An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 10:173–180
Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM (2006) Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res 98:1498–1505
Kwak BR, van Veen TA, Analbers LJ, Jongsma HJ (1995) TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junction channels. Exp Cell Res 220:456–463
Hofer A, Dermietzel R (1998) Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia 24:141–154
Dermietzel R, Hertberg EL, Kessler JA, Spray DC (1991) Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J Neurosci 11:1421–1432
Gosejacob D, Dublin P, Bedner P, Huttmann K, Zhang J, Tress O, Willecke K, Pfrieger F, Steinhauser C, Theis M (2011) Role of astroglial connexin30 in hippocampal gap junction coupling. Glia 59:511–519
Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C (2006) The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26:5438–5447
Orkand RK, Nicholls JG, Kuffler SW (1966) Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29:788–806
Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23:3588–3596
Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC (2002) Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99:495–500
Hansen DB, Ye ZC, Calloe K, Braunstein TH, Hofgaard JP, Ransom BR, Nielsen MS, MacAulay N (2014) Activation, permeability, and inhibition of astrocytic and neuronal large pore (hemi)channels. J Biol Chem 289:26058–26073
Morita M, Saruta C, Kozuka N, Okubo Y, Itakura M, Takahashi M, Kudo Y (2007) Dual regulation of astrocyte gap junction hemichannels by growth factors and a pro-inflammatory cytokine via the mitogen-activated protein kinase cascade. Glia 55:508–515
Orellana JA, Diaz E, Schalper KA, Vargas AA, Bennett MV, Saez JC (2011) Cation permeation through connexin 43 hemichannels is cooperative, competitive and saturable with parameters depending on the permeant species. Biochem Biophys Res Commun 409:603–609
Yi C, Mei X, Ezan P, Mato S, Matias I, Giaume C, Koulakoff A (2016) Astroglial connexin43 contributes to neuronal suffering in a mouse model of Alzheimer’s disease. Cell Death Differ 23:1691–1701
Abudara V, Roux L, Dallerac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C (2015) Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia 63:795–811
Gajardo-Gomez R, Labra VC, Maturana CJ, Shoji KF, Santibanez CA, Saez JC, Giaume C, Orellana JA (2017) Cannabinoids prevent the amyloid beta-induced activation of astroglial hemichannels: a neuroprotective mechanism. Glia 65:122–137
Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC, Bennett MV, Abudara V (2010) FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci USA 107:22659–22664
Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, Jiang JX, Naus CC, Saez JC, Giaume C (2011) Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J Neurosci 31:4962–4977
Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC (2006) S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103:4475–4480
Mei X, Ezan P, Giaume C, Koulakoff A (2010) Astroglial connexin immunoreactivity is specifically altered at beta-amyloid plaques in beta-amyloid precursor protein/presenilin1 mice. Neuroscience 171:92–105
Nagy JI, Li W, Hertzberg EL, Marotta CA (1996) Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res 717:173–178
Bukanova JV, Sharonova IN, Skrebitsky VG (2014) Amyloid beta peptide (25–35) in picomolar concentrations modulates the function of glycine receptors in rat hippocampal pyramidal neurons through interaction with extracellular site(s). Brain Res 1558:1–10
Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci 23:8844–8853
Giaume C, Theis M (2010) Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res Rev 63:160–176
Giaume C, Leybaert L, Naus CC, Saez JC (2013) Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol 4:88
Spray DC, Ye ZC, Ransom BR (2006) Functional connexin “hemichannels”: a critical appraisal. Glia 54:758–773
Aylsworth CF, Trosko JE, Welsch CW (1986) Influence of lipids on gap-junction-mediated intercellular communication between Chinese hamster cells in vitro. Cancer Res 46:4527–4533
Burt JM, Spray DC (1989) Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ Res 65:829–837
Burt JM (1989) Uncoupling of cardiac cells by doxyl stearic acids specificity and mechanism of action. Am J Physiol 256:C913–C924
Davidson JS, Baumgarten IM, Harley EH (1986) Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun 134:29–36
Davidson JS, Baumgarten IM (1988) Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther 246:1104–1107
Dhein S (1998) Gap junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends Pharmacol Sci 19:229–241
Guan X, Cravatt BF, Ehring GR, Hall JE, Boger DL, Lerner RA, Gilula NB (1997) The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J Cell Biol 139:1785–1792
Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP (2001) Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther 298:1033–1041
Srinivas M, Spray DC (2003) Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol 63:1389–1397
Juszczak GR, Swiergiel AH (2009) Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog Neuropsychopharmacol Biol Psychiatr 33:181–198
Patel D, Zhang X, Veenstra RD (2014) Connexin hemichannel and pannexin channel electrophysiology: How do they differ? FEBS Lett 588:1372–1378
Ye ZC, Oberheim N, Kettenmann H, Ransom BR (2009) Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57:258–269
Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M (2008) Connexin 43 hemichannels are permeable to ATP. J Neurosci 28:4702–4711
Brokamp C, Todd J, Montemagno C, Wendell D (2012) Electrophysiology of single and aggregate Cx43 hemichannels. PLoS ONE 7:e47775
Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C, Nobile M, Ferroni S (2009) Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels 3:323–336
Bruzzone R, Barbe MT, Jakob NJ, Monyer H (2005) Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92:1033–1043
Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2007) The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104:6436–6441
Qiu F, Dahl G (2009) A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol 296:C250–C255
Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L (2003) Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 33:37–48
Stout CE, Costantin JL, Naus CC, Charles AC (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277:10482–10488
Guinamard R, Simard C, Del NC (2013) Flufenamic acid as an ion channel modulator. Pharmacol Ther 138:272–284
Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD (2002) Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol 185:93–102
Hansen DB, Braunstein TH, Nielsen MS, MacAulay N (2014) Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Lett 588:1446–1457
Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F (2008) ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA 105:18770–18775
Bouron A, Kiselyov K, Oberwinkler J (2015) Permeation, regulation and control of expression of TRP channels by trace metal ions. Pflugers Arch 467:1143–1164
Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y (2008) Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res 18:558–565
Mlinar B, Enyeart JJ (1993) Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J Physiol 469:639–652
Yang XC, Sachs F (1989) Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 243:1068–1071
Dahl G, Nonner W, Werner R (1994) Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophys J 67:1816–1822
Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL (1995) Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol 488(Pt 3):721–728
Boitano S, Evans WH (2000) Connexin mimetic peptides reversibly inhibit Ca(2+) signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol 279:L623–L630
Isakson BE, Evans WH, Boitano S (2001) Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol 280:L221–L228
Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R (2012) Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res 65:546–552
Evans WH, Bultynck G, Leybaert L (2012) Manipulating connexin communication channels: use of peptidomimetics and the translational outputs. J Membr Biol 245:437–449
Wang N, De BM, Antoons G, Gadicherla AK, Bol M, Decrock E, Evans WH, Sipido KR, Bukauskas FF, Leybaert L (2012) Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol 107:304
Wang J, Ma M, Locovei S, Keane RW, Dahl G (2007) Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 293:C1112–C1119
Riquelme MA, Kar R, Gu S, Jiang JX (2013) Antibodies targeting extracellular domain of connexins for studies of hemichannels. Neuropharmacology 75:525–532
Ponsaerts R, De VE, Retamal M, D’hondt C, Vermeire D, Wang N, De SH, Zimmermann P, Himpens B, Vereecke J, Leybaert L, Bultynck G (2010) Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J 24:4378–4395
Wang N, De VE, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De BM, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L (2013) Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol 108:309
Abudara V, Bechberger J, Freitas-Andrade M, De BM, Wang N, Bultynck G, Naus CC, Leybaert L, Giaume C (2014) The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci 8:306
Wang N, De BM, Decrock E, Bol M, Gadicherla A, Bultynck G, Leybaert L (2013) Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening. Neuropharmacology 75:506–516
Clair C, Combettes L, Pierre F, Sansonetti P, Van Tran NG (2008) Extracellular-loop peptide antibodies reveal a predominant hemichannel organization of connexins in polarized intestinal cells. Exp Cell Res 314:1250–1265
Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX (2008) Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem 283:26374–26382
Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, DeSimone D, Bonewald LF, Lafer EM, Sprague E, Schwartz MA, Jiang JX (2012) Mechanical stress-activated integrin alpha5beta1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci USA 109:3359–3364
Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC (2011) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem 118:826–840
Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K (2008) The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet 17:539–554
Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K (2003) Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet 12:13–21
Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD (2007) A role for Connexin43 during neurodevelopment. Glia 55:675–686
Bol M, Wang N, De BM, Wacquier B, Decrock E, Gadicherla A, Decaluwe K, Vanheel B, van Rijen HV, Krysko DV, Bultynck G, Dupont G, Van de Voorde J, Leybaert L (2017) At the cross-point of connexins, calcium and ATP: blocking hemichannels inhibits vasoconstriction of rat small mesenteric arteries. Cardiovasc Res 113:195–206
Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR (2014) Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 137:2193–2209
Chever O, Lee CY, Rouach N (2014) Astroglial connexin43 hemichannels tune basal excitatory synaptic transmission. J Neurosci 34:11228–11232
Orellana JA, Saez JC, Bennett MV, Berman JW, Morgello S, Eugenin EA (2014) HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. J Neurochem 128:752–763
Orellana JA, Busso D, Ramirez G, Campos M, Rigotti A, Eugenin J, von BR (2014) Prenatal nicotine exposure enhances Cx43 and Panx1 unopposed channel activity in brain cells of adult offspring mice fed a high-fat/cholesterol diet. Front Cell Neurosci 8:403
Orellana JA, Avendano BC, Montero TD (2014) Role of connexins and pannexins in ischemic stroke. Curr Med Chem 21:2165–2182
Retamal MA, Alcayaga J, Verdugo CA, Bultynck G, Leybaert L, Saez PJ, Fernandez R, Leon LE, Saez JC (2014) Opening of pannexin- and connexin-based channels increases the excitability of nodose ganglion sensory neurons. Front Cell Neurosci 8:158
Rovegno M, Soto PA, Saez PJ, Naus CC, Saez JC, von BR (2015) Connexin43 hemichannels mediate secondary cellular damage spread from the trauma zone to distal zones in astrocyte monolayers. Glia 63:1185–1199
Iacobas DA, Iacobas S, Urban-Maldonado M, Spray DC (2005) Sensitivity of the brain transcriptome to connexin ablation. Biochim Biophys Acta 1711:183–196
Iacobas DA, Iacobas S, Urban-Maldonado, M, Scemes E, Spray DC (2008) Similar transcriptomic alterations in Cx43 knockdown and knockout astrocytes. Cell Commun Adhes 15:195–206
Jansen JA, Noorman M, Musa H, Stein M, de JS, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV (2012) Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm 9:600–607
Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Liang FX, Li Z, Pfenniger A, Lubkemeier I, Keegan S, Fenyo D, Willecke K, Rothenberg E, Delmar M (2014) Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and NaV1.5 localization at the intercalated disc. Cardiovasc Res 104:371–381
May D, Tress O, Seifert G, Willecke K (2013) Connexin47 protein phosphorylation and stability in oligodendrocytes depend on expression of Connexin43 protein in astrocytes. J Neurosci 33:7985–7996
Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E (2009) Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29:7092–7097
Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG (1996) Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol 134:1019–1030
Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MV, Nicholson BJ (1991) Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci USA 88:8410–8414
Bao X, Altenberg GA, Reuss L (2004) Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol 286:C647–C654
Cao F, Eckert R, Elfgang C, Nitsche JM, Snyder SA, ulser DF, Willecke K, Nicholson BJ (1998) A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J Cell Sci 111(Pt 1):31–43
Dahl G, Miller T, Paul D, Voellmy R, Werner R (1987) Expression of functional cell-cell channels from cloned rat liver gap junction complementary DNA. Science 236:1290–1293
Ek-Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M (1996) PH regulation of connexin43: molecular analysis of the gating particle. Biophys J 71:1273–1284
DeVuyst E, Wang N, Decrock E, De BM, Vinken M, Van MM, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L (2009) Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 46:176–187
Ramachandran, S., Xie, L.H., John, S.A., Subramaniam S, Lal R (2007) A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS ONE. 2:e712
Alstrom JS, Hansen DB, Nielsen MS, MacAulay N (2015) Isoform-specific phosphorylation-dependent regulation of connexin hemichannels. J Neurophysiol 114:3014–3022
Valiunas V, Weingart R (2000) Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Arch 440:366–379
Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G (2004) Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum Mol Genet 13:1703–1714
Contreras JE, Saez JC, Bukauskas FF, Bennett MV (2003) Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA 100:11388–11393
Valiunas V (2013) Cyclic nucleotide permeability through unopposed connexin hemichannels. Front Pharmacol 4:75
Saez JC, Leybaert L (2014) Hunting for connexin hemichannels. FEBS Lett 588:1205–1211
Retamal MA, Froger N, Palacios-Prado N, Ezan P, Saez PJ, Saez JC, Giaume C (2007) Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27:13781–13792
John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN (1999) Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274:236–240
Fasciani I, Temperan A, Perez-Atencio LF, Escudero A, Martinez-Montero P, Molano J, Gomez-Hernandez JM, Paino CL, Gonzalez-Nieto D, Barrio LC (2013) Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology 75:479–490
Ebihara L (1996) Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys J 71:742–748
Scemes E, Spray DC, Meda P (2009) Connexins, pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch 457:1207–1226
Stroud RM, Savage D, Miercke LJ, Lee JK, Khademi S, Harries W (2003) Selectivity and conductance among the glycerol and water conducting aquaporin family of channels. FEBS Lett 555:79–84
Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, van Hoek AN, Hediger MA (1998) Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem 273:24737–24743
Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T (2009) Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458:597–602
Unger VM, Kumar NM, Gilula NB, Yeager M (1999) Three-dimensional structure of a recombinant gap junction membrane channel. Science 283:1176–1180
Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M (2000) ATP-mediated glia signaling. J Neurosci 20:2835–2844
Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M (2012) Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 60:1660–1670
Huang C, Han X, Li X, Lam E, Peng W, Lou N, Torres A, Yang M, Garre JM, Tian GF, Bennett MV, Nedergaard M, Takano T (2012) Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. J Neurosci 32:3333–3338
Langevin HM, Fujita T, Bouffard NA, Takano T, Koptiuch C, Badger GJ, Nedergaard M (2013) Fibroblast cytoskeletal remodeling induced by tissue stretch involves ATP signaling. J Cell Physiol 228:1922–1926
Stridh MH, Tranberg M, Weber SG, Blomstrand F, Sandberg M (2008) Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J Biol Chem 283:10347–10356
Ye B, Shen H, Zhang J, Zhu YG, Ransom BR, Chen XC, Ye ZC (2015) Dual pathways mediate beta-amyloid stimulated glutathione release from astrocytes. Glia 63:2208–2219
Zhang J, Zhang HY, Zhang M, Qiu ZY, Wu YP, Callaway DA, Jiang JX, Lu L, Jing L, Yang T, Wang MQ (2014) Connexin43 hemichannels mediate small molecule exchange between chondrocytes and matrix in biomechanically-stimulated temporomandibular joint cartilage. Osteoarthritis Cartil 22:822–830
Fellin T, Pozzan T, Carmignoto G (2006) Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem 281:4274–4284
Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ (2006) Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia 54:343–357
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281:21362–21368
Fenton RA, Moeller HB, Zelenina M, Snaebjornsson MT, Holen T, MacAulay N (2010) Differential water permeability and regulation of three aquaporin 4 isoforms. Cell Mol Life Sci 67:829–840
Joshi-Mukherjee R, Coombs W, Burrer C, de Mora IA, Delmar M, Taffet SM (2007) Evidence for the presence of a free C-terminal fragment of cx43 in cultured cells. Cell Commun Adhes 14:75–84
Giaume, C., Orellana, J.A., Abudara, V., Saez, J.C. 2012. Connexin-based channels in astrocytes: how to study their properties. In Milner R (ed), Astrocytes methods in molecular biology, Springer Protocols, Berlin, pp 283–303
Schalper KA, Palacios-Prado N, Orellana JA, Saez, JC (2008) Currently used methods for identification and characterization of hemichannels. Cell Commun Adhes 15:207–218
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2 × 7 receptor. EMBO J 25:5071–5082
Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E (2008) P2 × 7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295:C752–C760
Nakazawa K, Liu M, Inoue K, Ohno Y (1997) Potent inhibition by trivalent cations of ATP-gated channels. Eur J Pharmacol 325:237–243
Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, de Groat WC (2015) Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol 593:1857–1871
Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS (2010) Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299:H1146–H1152
Suadicani SO, Brosnan CF, Scemes E (2006) P2 × 7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 26:1378–1385
Tovar KR, Maher BJ, Westbrook GL (2009) Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J Neurophysiol 102:974–978
Braet K, Aspeslagh S, Vandamme W, Willecke K, Martin PE, Evans WH, Leybaert L (2003) Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J Cell Physiol 197:205–213
Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S (2004) Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol 92:1252–1256
Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER (2011) Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286:26277–26286
Li K, Chi Y, Gao K, Yan Q, Matsue H, Takeda M, Kitamura M, Yao J (2013) Connexin43 hemichannel-mediated regulation of connexin43. PLoS ONE 8:e58057
Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2 × (7) receptor death complex. FEBS Lett 581:483–488
Rae MG, Hilton J, Sharkey J (2012) Putative TRP channel antagonists, SKF 96365, flufenamic acid and 2-APB, are non-competitive antagonists at recombinant human alpha1beta2gamma2 GABA(A) receptors. Neurochem Int 60:543–554
Woodward RM, Polenzani L, Miledi R (1994) Effects of fenamates and other nonsteroidal anti-inflammatory drugs on rat brain GABAA receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 268:806–817
Hu H, Tian J, Zhu Y, Wang C, Xiao R, Herz JM, Wood JD, Zhu MX (2010) Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch 459:579–592
McCarty NA, McDonough S, Cohen BN, Riordan JR, Davidson N, Lester HA (1993) Voltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl- channel by two closely related arylaminobenzoates. J Gen Physiol 102:1–23
Ma W, Hui H, Pelegrin P, Surprenant A (2009) Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 328:409–418
Nathan RD, Kanai K, Clark RB, Giles W (1988) Selective block of calcium current by lanthanum in single bullfrog atrial cells. J Gen Physiol 91:549–572
Wei H, Deng F, Chen Y, Qin Y, Hao Y, Guo X (2014) Ultrafine carbon black induces glutamate and ATP release by activating connexin and pannexin hemichannels in cultured astrocytes. Toxicology 323:32–41
Funding
This study was supported by Sundhed og Sygdom, Det Frie Forskningsråd (Grant No. 10-081105).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nielsen, B.S., Hansen, D.B., Ransom, B.R. et al. Connexin Hemichannels in Astrocytes: An Assessment of Controversies Regarding Their Functional Characteristics. Neurochem Res 42, 2537–2550 (2017). https://doi.org/10.1007/s11064-017-2243-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2243-7