Abstract
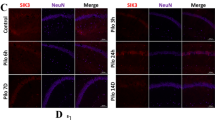
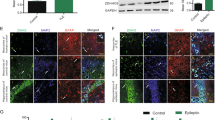
FK506, a calcineurin inhibitor, shows neuroprotective effects and has been associated with neurodegenerative diseases. Calcineurin A (CaNA), a catalytic subunit of calcineurin, mediates the dephosphorylation of various proteins. N-methyl-d-aspartate receptor (GluN) is closely related to epileptogenesis, and various phosphorylation sites of GluN2B, a regulatory subunit of the GluN complex, have different functions. Thus, we hypothesized that one of the potential anti-epileptic mechanisms of FK506 is mediated by its ability to promote the phosphorylation of GluN2B and reduce the expression of GluN2B in membrane fraction by down-regulating CaNA. CaNA expression was increased in the cortex of patients with temporal lobe epilepsy and pentylenetetrazol (PTZ)-induced epileptic models. CaNA was shown to be expressed in neurons using immunofluorescence staining. According to our behavioral observations, epileptic rats exhibited less severe seizures and were less sensitive to PTZ after a systemic injection of FK506. The levels of phosphorylated GluN2B were decreased in epileptic rats but increased after the FK506 treatment. Moreover, there was no difference in the total GluN2B levels before and after FK506 treatment. However, the expression of GluN2B in membrane fraction was suppressed after FK506 treatment. Based on these results, FK506 may reduce the severity and frequency of seizures by reducing the expression of GluN2B in membrane fraction.





Similar content being viewed by others
Abbreviations
- CaN:
-
Calcineurin
- CaNA:
-
Calcineurin A
- GluN:
-
N-methyl-d-aspartate receptor
- TLE:
-
Temporal lobe epilepsy.
- PTZ:
-
Pentylenetetrazol
- SRS:
-
Spontaneous recurrent seizures
- GluRs:
-
Glutamate receptors
- CaM:
-
Calmodulin
- CREB:
-
cAMP response element-binding protein
- NFAT:
-
Nuclear factor of activated T cells
- BAD:
-
Bcl2-associated death promoter
- EPSCs:
-
Excitatory postsynaptic currents
- CsA:
-
Cyclosporin A
- BDNF:
-
Brain-derived neurotrophic factor
- GluA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- KA:
-
Kainic acid
References
Singh A, Trevick S (2016) The epidemiology of global epilepsy. Neurol Clin 34(4):837–847. doi:10.1016/j.ncl.2016.06.015
Babb TL, Kupfer WR, Pretorius JK et al (1991) Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience 42(2):351–363
Fisher RS, van Emde Boas W, Blume W et al (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46(4):470–472. doi:10.1111/j.0013-9580.2005.66104.x
Weiner JL, Buhler AV, Whatley VJ et al (1998) Colchicine is a competitive antagonist at human recombinant gamma-aminobutyric acidA receptors. J Pharmacol Exp Ther 284(1):95–102
Missiaen L, Robberecht W, van den Bosch L et al (2000) Abnormal intracellular Ca(2+)homeostasis and disease. Cell Calcium 28(1):1–21. doi:10.1054/ceca.2000.0131
Isokawa M (2005) N-methyl-D-aspartic acid-induced and Ca-dependent neuronal swelling and its retardation by brain-derived neurotrophic factor in the epileptic hippocampus. Neuroscience 131(4):801–812. doi:10.1016/j.neuroscience.2004.12.008
Yilmaz M, Naziroğlu M, Kutluhan S et al (2011) Topiramate modulates hippocampus NMDA receptors via brain Ca(2+) homeostasis in pentylentetrazol-induced epilepsy of rats. J Recept Signal Transduct Res 31(2):173–179. doi:10.3109/10799893.2011.555914
Shah SZ, Hussain T, Zhao D et al (2016) A central role for calcineurin in protein misfolding neurodegenerative diseases. Cell Mol Life Sci. doi:10.1007/s00018-016-2379-7
Lieberman DN, Mody I (1994) Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature 369(6477):235–239. doi:10.1038/369235a0
Fukuta T, Ishii T, Asai T et al (2015) Treatment of stroke with liposomal neuroprotective agents under cerebral ischemia conditions. Eur J Pharm Biopharm 97(Pt A):1–7. doi:10.1016/j.ejpb.2015.09.020
Zawadzka M, Dabrowski M, Gozdz A et al (2012) Early steps of microglial activation are directly affected by neuroprotectant FK506 in both in vitro inflammation and in rat model of stroke. J Mol Med 90(12):1459–1471. doi:10.1007/s00109-012-0925-9
Rozkalne A, Hyman BT, Spires-Jones TL (2011) Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis 41(3):650–654. doi:10.1016/j.nbd.2010.11.014
Quintanilla RA, Jin YN, von Bernhardi R (2013) Mitochondrial permeability transition pore induces mitochondria injury in Huntington disease. Mol Neurodegener 8:45. doi:10.1186/1750-1326-8-45
Seeburg PH (1993) The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci 16(9):359–365
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–294
Nishimura T, Imai H, Minabe Y et al (2006) Beneficial effects of FK506 for experimental temporal lobe epilepsy. Neurosci Res 56(4):386–390. doi:10.1016/j.neures.2006.08.006
Fang M, Wei JL, Tang B et al (2016) Neuroligin-1 knockdown suppresses seizure activity by regulating neuronal hyperexcitability. Mol Neurobiol 53(1):270–284. doi:10.1007/s12035-014-8999-8
Goto S, Matsukado Y, Mihara Y et al (1986) The distribution of calcineurin in rat brain by light and electron microscopic immunohistochemistry and enzyme-immunoassay. Brain Res 397(1):161–172
Goto S, Matsukado Y, Mihara Y et al (1986) Calcineurin in human brain and its relation to extrapyramidal system. Immunohistochemical study on postmortem human brains. Acta Neuropathol 72(2):150–156
Bito H, Deisseroth K, Tsien RW (1996) CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87(7):1203–1214
Kingsbury TJ, Bambrick LL, Roby CD et al (2007) Calcineurin activity is required for depolarization-induced, CREB-dependent gene transcription in cortical neurons. J Neurochem 103(2):761–770. doi:10.1111/j.1471-4159.2007.04801.x
Wei Q, Holzer M, Brueckner MK et al (2002) Dephosphorylation of tau protein by calcineurin triturated into neural living cells. Cell Mol Neurobiol 22(1):13–24
Yin Y, Gao D, Wang Y et al (2016) Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci USA 113(26):E3773–E3781. doi:10.1073/pnas.1604519113
Minami T (2014) Calcineurin-NFAT activation and DSCR-1 auto-inhibitory loop: how is homoeostasis regulated? J Biochem 155(4):217–226. doi:10.1093/jb/mvu006
Wang J, Wang Y, Zhang W et al (2016) Phenylephrine promotes cardiac fibroblast proliferation through calcineurin-NFAT pathway. Front Biosci 21:502–513
Wu HY, Hudry E, Hashimoto T et al (2010) Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci 30(7):2636–2649. doi:10.1523/JNEUROSCI.4456-09.2010
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245. doi:10.1146/annurev.biochem.69.1.217
Liu F, Grundke-Iqbal I, Iqbal K et al (2005) Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem 280(45):37755–37762. doi:10.1074/jbc.M507475200
Dineley KT, Kayed R, Neugebauer V et al (2010) Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res 88(13):2923–2932. doi:10.1002/jnr.22445
Hong HS, Hwang JY, Son SM et al (2010) FK506 reduces amyloid plaque burden and induces MMP-9 in AβPP/PS1double transgenic mice. J Alzheimers Dis 22(1):97–105. doi:10.3233/JAD-2010-100261
Kawakami M, Yoshimoto T, Nakagata N et al (2011) Effects of cyclosporin A administration on gene expression in rat brain. Brain Inj 25(6):614–623. doi:10.3109/02699052.2011.571229
Overk CR, Rockenstein E, Florio J et al (2015) Differential calcium alterations in animal models of neurodegenerative disease: reversal by FK506. Neuroscience 310:549–560. doi:10.1016/j.neuroscience.2015.08.068
Lakshmikuttyamma A, Selvakumar P, Tuchek J et al (2008) Myristoyltransferase and calcineurin: novel molecular therapeutic target for epilepsy. Prog Neurobiol 84(1):77–84. doi:10.1016/j.pneurobio.2007.09.004
Lie AA, Blümcke I, Beck H et al (1998) Altered patterns of Ca2+/calmodulin-dependent protein kinase II and calcineurin immunoreactivity in the hippocampus of patients with temporal lobe epilepsy. J Neuropathol Exp Neurol 57(11):1078–1088
Ingram EA, Toyoda I, Wen X et al (2009) Prolonged infusion of inhibitors of calcineurin or L-type calcium channels does not block mossy fiber sprouting in a model of temporal lobe epilepsy. Epilepsia 50(1):56–64. doi:10.1111/j.1528-1167.2008.01704.x
Koyama R, Ikegaya Y (2004) Mossy fiber sprouting as a potential therapeutic target for epilepsy. Curr Neurovasc Res 1(1):3–10
Proper EA, Oestreicher AB, Jansen GH et al (2000) Immunohistochemical characterization of mossy fibre sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 123(Pt1):19–30
Fang M, Xi ZQ, Wu Y et al (2011) A new hypothesis of drug refractory epilepsy: neural network hypothesis. Med Hypotheses 76(6):871–876. doi:10.1016/j.mehy.2011.02.039
Thomas U, Sigrist SJ (2012) Glutamate receptors in synaptic assembly and plasticity: case studies on fly NMJs. Adv Exp Med Biol 970:3–28. doi:10.1007/978-3-7091-0932-8
Manabe T (2004) Synaptic plasticity and NMDA-type glutamate receptors. Tanpakushitsu Kakusan Koso 49(3 Suppl):398–404
Upreti C, Zhang XL, Alford S et al (2013) Role of presynaptic metabotropic glutamate receptors in the induction of long-term synaptic plasticity of vesicular release. Neuropharmacology 66:31–39. doi:10.1016/j.neuropharm.2012.05.004
Huettner JE (2015) Glutamate receptor pores. J Physiol 593(1):49–59. doi:10.1113/jphysiol.2014.272724
Sachser RM, Santana F, Crestani AP et al (2016) Forgetting of long-term memory requires activation of NMDA receptors, L-type voltage-dependent Ca2+ channels, and calcineurin. Sci Rep 6:22771. doi:10.1038/srep22771
Kim S, Violette CJ, Ziff EB (2015) Reduction of increased calcineurin activity rescues impaired homeostatic synaptic plasticity in presenilin 1 M146V mutant. Neurobiol. Aging 36(12):3239–3246. doi:10.1016/j.neurobiolaging.2015.09.007
Kim S, Ziff EB (2014) Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biol 12(7):e1001900. doi:10.1371/journal.pbio.1001900
Kam AY, Liao D, Loh HH et al (2010) Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci 30(45):15304–15316. doi:10.1523/JNEUROSCI.4255-10.2010
Zhao WQ, Santini F, Breese R et al (2010) Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem 285(10):7619–7632. doi:10.1074/jbc.M109.057182
Halt AR, Dallapiazza RF, Zhou Y et al (2012) CaMKII binding to GluN2B is critical during memory consolidation. EMBO J 31(5):1203–1216. doi:10.1038/emboj.2011.482
Sanhueza M, Fernandez-Villalobos G, Stein IS et al (2011) Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci 31(25):9170–9178. doi:10.1523/JNEUROSCI.1250-11.2011
Tu W, Xu X, Peng L et al (2010) DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 140(2):222–234. doi:10.1016/j.cell.2009.12.055
Liao GY, Wagner DA, Hsu MH et al (2001) Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol Pharmacol 59(5):960–964
Sanz-Clemente A, Matta JA, Isaac JT et al (2010) Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67(6):984–996. doi:10.1016/j.neuron.2010.08.011
Prybylowski K, Chang K, Sans N et al (2005) The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 47(6):845–857. doi:10.1016/j.neuron.2005.08.016
Chung HJ, Huang YH, Lau LF et al (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 24(45):10248–10259. doi:10.1523/JNEUROSCI.0546-04.2004
Acknowledgements
This work was supported by the Foundation of Chongqing Health Bureau (2013-1-005). We sincerely thank the support of Chongqing Key Laboratory of Neurology, which provided the brain tissue samples. We also thank the patients and families for the donation of brains.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standareds of the Chongqing Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standareds.
Rights and permissions
About this article
Cite this article
Wen, Y., Fu, P., Wu, K. et al. Inhibition of Calcineurin A by FK506 Suppresses Seizures and Reduces the Expression of GluN2B in Membrane Fraction. Neurochem Res 42, 2154–2166 (2017). https://doi.org/10.1007/s11064-017-2221-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2221-0