Abstract
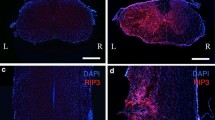
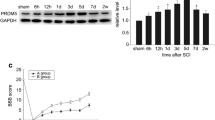
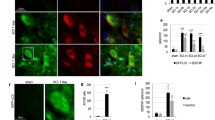
Spinal cord injury (SCI) is one of the most common and severe complications in spine injury. It is difficult to prevent cell necroptosis and promote the survival of residual neurons after SCI. Proteasome beta-4 subunit (PSMB4) is the first proteasomal subunit with oncogenic properties promoting cancer cell survival and tumor growth in vivo, and our previous study showed that PSMB4 is significantly associated with neuronal apoptosis in neuroinflammation. However, PSMB4 function in the necroptosis after SCI is unkown. RIP3, a key regulatory factor of necroptosis, correlates with the induction of necroptosis in various types of cells and signaling pathway. Upregulation of the RIP3 expression may play a role as a novel molecular mechanism in secondary neural tissue damage following SCI. In this study, we established an acute spinal cord contusion injury model in adult rats to investigate the potential role of PSMB4 during the pathological process of SCI. We found PSMB4 expression was significantly up-regulated 3 days after injury by western blot and immunohistochemical staining. Double immunofluorescent staining indicated obvious changes of PSMB4 expression occurred in neurons. Significant up-regulation of PSMB4 expression was observed in Rip3 positive neurons at 3 days after SCI, which indicated that PSMB4 might play a vital role in the regulation of Rip3. Overexpress and knockdown PSMB4 could intervene the RIP3 and Mixed lineage kinase domain-like protein (MLKL) pathway in Tumor necrosis factor-α (TNF-α) induced necroptosis cell model. Based on our experimental data, we boldly conclude that PSMB4 is associated with RIP3 involved necroptosis after SCI.




Similar content being viewed by others
References
Selvarajah S, Hammond ER, Schneider EB (2015) Trends in traumatic spinal cord injury. JAMA 314(15):1643. doi:10.1001/jama.2015.11194
Sawada M, Kato K, Kunieda T, Mikuni N, Miyamoto S, Onoe H, Isa T, Nishimura Y (2015) Function of the nucleus accumbens in motor control during recovery after spinal cord injury. Science 350(6256):98–101. doi:10.1126/science.aab3825
Zhang J, Cui Z, Feng G, Bao G, Xu G, Sun Y, Wang L, Chen J, Jin H, Liu J, Yang L, Li W (2015) RBM5 and p53 expression after rat spinal cord injury: implications for neuronal apoptosis. Int J Biochem Cell Biol 60:43–52. doi:10.1016/j.biocel.2014.12.020
Fan H, Zhang K, Shan L, Kuang F, Chen K, Zhu K, Ma H, Ju G, Wang YZ (2016) Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Molecular Neurodegener 11(1):14. doi:10.1186/s13024-016-0081-8
Liu M, Wu W, Li H, Li S, Huang LT, Yang YQ, Sun Q, Wang CX, Yu Z, Hang CH (2015) Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J Spinal Cord Med 38(6):745–753. doi:10.1179/2045772314Y.0000000224
Liu T, Zhao DX, Cui H, Chen L, Bao YH, Wang Y, Jiang JY (2016) Therapeutic hypothermia attenuates tissue damage and cytokine expression after traumatic brain injury by inhibiting necroptosis in the rat. Scientific Rep 6:24547. doi:10.1038/srep24547
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119. doi:10.1038/nchembio711
You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, Moskowitz MA, Whalen MJ (2008) Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 28(9):1564–1573. doi:10.1038/jcbfm.2008.44
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135(7):1311–1323. doi:10.1016/j.cell.2008.10.044
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4(5):313–321. doi:10.1038/nchembio.83
Montgomery SL, Bowers WJ (2012) Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol 7(1):42–59. doi:10.1007/s11481-011-9287-2
Liu S, Wang X, Li Y, Xu L, Yu X, Ge L, Li J, Zhu Y, He S (2014) Necroptosis mediates TNF-induced toxicity of hippocampal neurons. Biomed Res Int 2014:290182. doi:10.1155/2014/290182
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19(1):107–120. doi:10.1038/cdd.2011.96
Han J, Zhong CQ, Zhang DW (2011) Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol 12(12):1143–1149. doi:10.1038/ni.2159
Moriwaki K, Chan FK (2013) RIP3: a molecular switch for necrosis and inflammation. Genes Dev 27(15):1640–1649. doi:10.1101/gad.223321.113
Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE (2013) Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57(5):1773–1783. doi:10.1002/hep.26200
Khan N, Lawlor KE, Murphy JM, Vince JE (2014) More to life than death: molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr Opin Immunol 26:76–89. doi:10.1016/j.coi.2013.10.017
Kanno H, Ozawa H, Tateda S, Yahata K, Itoi E (2015) Upregulation of the receptor-interacting protein 3 expression and involvement in neural tissue damage after spinal cord injury in mice. BMC Neurosci 16:62. doi:10.1186/s12868-015-0204-0
Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM (2002) Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem 277(11):9505–9511. doi:10.1074/jbc.M109488200
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148(1–2):213–227. doi:10.1016/j.cell.2011.11.031
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA 109(14):5322–5327. doi:10.1073/pnas.1200012109
Zapatero A, Morente M, Nieto S, Martin de Vidales C, Lopez C, Adrados M, Arellano R, Artiga MJ, Garcia-Vicente F, Herranz LM, Leaman O (2014) Predictive value of PAK6 and PSMB4 expression in patients with localized prostate cancer treated with dose-escalation radiation therapy and androgen deprivation therapy. Urol Oncol 32(8):1327–1332. doi:10.1016/j.urolonc.2014.05.004
Wong ML, Dong C, Maestre-Mesa J, Licinio J (2008) Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry 13(8):800–812. doi:10.1038/mp.2008.59
Zheng P, Guo H, Li G, Han S, Luo F, Liu Y (2015) PSMB4 promotes multiple myeloma cell growth by activating NF-kappaB-miR-21 signaling. Biochem Biophys Res Commun 458(2):328–333. doi:10.1016/j.bbrc.2015.01.110
Moriwaki K, Chan FK (2016) Regulation of RIPK3- and RHIM-dependent Necroptosis by the Proteasome. J Biol Chem 291(11):5948–5959. doi:10.1074/jbc.M115.700997
Hirano Y, Kaneko T, Okamoto K, Bai M, Yashiroda H, Furuyama K, Kato K, Tanaka K, Murata S (2008) Dissecting beta-ring assembly pathway of the mammalian 20 S proteasome. EMBO J 27(16):2204–2213. doi:10.1038/emboj.2008.148
Murata S, Yashiroda H, Tanaka K (2009) Molecular mechanisms of proteasome assembly. Nat Rev Mol Cell Biol 10(2):104–115. doi:10.1038/nrm2630
Lee GY, Haverty PM, Li L, Kljavin NM, Bourgon R, Lee J, Stern H, Modrusan Z, Seshagiri S, Zhang Z, Davis D, Stokoe D, Settleman J, de Sauvage FJ, Neve RM (2014) Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. Cancer Res 74(11):3114–3126. doi:10.1158/0008-5472.CAN-13-2683
Zhang J, Jin Z, Du Q, Li R, Yao F, Huang B, Xu N, Xu L, Luo X, Liu X (2012) Analysis of altered proteins related to blast crisis in chronic myeloid leukemia by proteomic study. Int J Lab Hematol 34(3):267–273. doi:10.1111/j.1751-553X.2011.01389.x
Thaker NG, Zhang F, McDonald PR, Shun TY, Lewen MD, Pollack IF, Lazo JS (2009) Identification of survival genes in human glioblastoma cells by small interfering RNA screening. Mol Pharmacol 76(6):1246–1255. doi:10.1124/mol.109.058024
Cui F, Wang Y, Wang J, Wei K, Hu J, Liu F, Wang H, Zhao X, Zhang X, Yang X (2006) The up-regulation of proteasome subunits and lysosomal proteases in hepatocellular carcinomas of the HBx gene knockin transgenic mice. Proteomics 6(2):498–504. doi:10.1002/pmic.200500218
Shi J, Liu X, Xu C, Ge J, Ren J, Wang J, Song X, Dai S, Tao W, Lu H (2015) Up-regulation of PSMB4 is associated with neuronal apoptosis after neuroinflammation induced by lipopolysaccharide. J Mol Histol 46(6):457–466. doi:10.1007/s10735-015-9637-0
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Obuchowicz E, Kowalski J, Labuzek K, Krysiak R, Pendzich J, Herman ZS (2006) Amitriptyline and nortriptyline inhibit interleukin-1 release by rat mixed glial and microglial cell cultures. Int J Neuropsychopharmacol 9(1):27–35. doi:10.1017/S146114570500547X
Das A, Smith JA, Gibson C, Varma AK, Ray SK, Banik NL (2011) Estrogen receptor agonists and estrogen attenuate TNF-alpha-induced apoptosis in VSC4.1 motoneurons. J Endocrinol 208(2):171–182. doi:10.1677/JOE-10-0338
Oliver Metzig M, Fuchs D, Tagscherer KE, Grone HJ, Schirmacher P, Roth W (2015) Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-alpha-dependent necroptosis driven by RIP1 kinase and NF-kappaB. Oncogene. doi:10.1038/onc.2015.398
Zhao XM, Chen Z, Zhao JB, Zhang PP, Pu YF, Jiang SH, Hou JJ, Cui YM, Jia XL, Zhang SQ (2016) Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis 7:e2089. doi:10.1038/cddis.2015.390
Wang Z, Jiang H, Chen S, Du F, Wang X (2012) The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148(1–2):228–243. doi:10.1016/j.cell.2011.11.030
Acknowledgments
This work was supported by grants from the Nantong University (Grant Codes: YKC15095) and Spine Surgery Clinical Medical Research Center (Grant Codes: HS2014002). We appreciate the technical assistances from M.M. Fang Liu in Jiangsu Province Key Laboratory of neural regeneration. We thank M.D. Aiguo Shen (Nantong University) for editing the language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Chunshuai Wu and Jiajia Chen have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, C., Chen, J., Liu, Y. et al. Upregulation of PSMB4 is Associated with the Necroptosis after Spinal Cord Injury. Neurochem Res 41, 3103–3112 (2016). https://doi.org/10.1007/s11064-016-2033-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2033-7