Abstract
Mammalian glutamate dehydrogenase1 (GDH1) (E.C. 1.4.1.3) is a mitochondrial enzyme that catalyzes the reversible oxidative deamination of glutamate to α-ketoglutarate and ammonia while reducing NAD+ and/or NADP+ to NADH and/or NADPH. It links amino acid with carbohydrate metabolism, contributing to Krebs cycle anaplerosis, energy production, ammonia handling and redox homeostasis. Although GDH1 was one of the first major metabolic enzymes to be studied decades ago, its role in cell biology is still incompletely understood. There is however growing interest in a novel GDH2 isoenzyme that emerged via duplication in primates and underwent rapid evolutionary selection concomitant with prefrontal human cortex expansion. Also, the anaplerotic function of GDH1 and GDH2 is currently under sharp focus as this relates to the biology of glial tumors and other neoplasias. Here we used antibodies specific for human GDH1 (hGDH1) and human GDH2 (hGDH2) to study the expression of these isoenzymes in human tissues. Results revealed that both hGDH1 and hGDH2 are expressed in human brain, kidney, testis and steroidogenic organs. However, distinct hGDH1 and hGDH2 expression patterns emerged. Thus, while the Sertoli cells of human testis were strongly positive for hGDH2, they were negative for hGDH1. Conversely, hGDH1 showed very high levels of expression in human liver, but hepatocytes were virtually devoid of hGDH2. In human adrenals, both hGDHs were densely expressed in steroid-producing cells, with hGDH2 expression pattern matching that of the cholesterol side chain cleavage system involved in steroid synthesis. Similarly in human ovaries and placenta, both hGDH1 and hGDH2 were densely expressed in estrogen producing cells. In addition, hGDH1, being a housekeeping enzyme, was also expressed in cells that lack endocrine function. Regarding human brain, study of cortical sections using immunofluorescence (IF) with confocal microscopy revealed that hGDH1 and hGDH2 were both expressed in the cytoplasm of gray and white matter astrocytes within coarse structures resembling mitochondria. Additionally, hGDH1 localized to the nuclear membrane of a subpopulation of astrocytes and of the vast majority of oligodendrocytes and their precursors. Remarkably, hGDH2-specific staining was detected in human cortical neurons, with different expression patterns having emerged. One pattern, observed in large cortical neurons (some with pyramidal morphology), was a hGDH2-specific labeling of cytoplasmic structures resembling mitochondria. These were distributed either in the cell body-axon or on the cell surface in close proximity to astrocytic end-feet that encircle glutamatergic synapses. Another pattern was observed in small cortical neurons with round dense nuclei in which the hGDH2-specific staining was found in the nuclear membrane. A detailed description of these observations and their functional implications, suggesting that the GDH flux is used by different cells to serve some of their unique functions, is presented below.

(Modified from [45])

(Modified from [45])

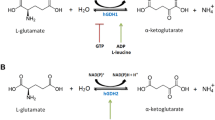
(Modified from [56])

(Modified from [56])

(Modified from [56])

(Modified from [56])

(Modified from [56])
Similar content being viewed by others
References
Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, Boggon TJ, Jin P, Yi H, Wright ER, Duong D, Seyfried NT, Egnatchik R, DeBerardinis RJ, Magliocca KR, He C, Arellano ML, Khoury HJ, hin DM, Khuri FR, Kang S (2015) Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 27:257–270
Krebs HA, Gascoyne T (1968) The redox state of the nicotinamide-adenine dinucleotides in rat liver homogenates. Biochem J 108:513–520
Cooper AJ, Nieves E, Coleman AE, Filc-DeRicco S, Gelbard AS (1987) Short-term metabolic fate of [13N] ammonia in rat liver in vivo. J Biol Chem 262:1073–1080
Cooper AJ, Nieves E, Rosenspire KC, Filc-DeRicco S, Gelbard AS, Brusilow SW (1988) Short-term metabolic fate of 13 N-labeled glutamate, alanine, and glutamine (amide) in rat liver. J Biol Chem 263:12268–12273
Schoolwerth AC, Nazar BL, LaNoue KF (1978) Glutamate dehydrogenase activation and ammonia formation by rat kidney mitochondria. J Biol Chem 253:6177–6183
Sener A, Malaisse WJ (1980) l-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature 288:187–189
Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, Perlman K, Rich BH, Zammarchi E, Poncz M (1998) Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 338:1352–1357
Cooper AJ (2011) 13N as a tracer for studying glutamate metabolism. Neurochem Int 59:456–464
Berl S, Takagaki G, Clarke DD, Waelsch H (1962) Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem 237:2562–2569
Kanamori K, Ross BD (1995) Steady-state in vivo glutamate dehydrogenase activity in rat brain measured by 15 N NMR. J Biol Chem 270:24805–24809
Dadsetan S, Kukolj E, Bak LK, Sørensen M, Ott P, Vilstrup H, Schousboe A, Keiding S, Waagepetersen HS (2013) Brain alanine formation as an ammonia-scavenging pathway during hyperammonemia: effects of glutamine synthetase inhibition in rats and astrocyte-neuron co-cultures. J Cereb Blood Flow Metab 33:1235–1241
Cooper AJ, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE (1979) The metabolic fate of 13 N-labeled ammonia in rat brain. J Biol Chem 254:4982–4992
Nadler JV, White WF, Vaca KW, Perry BW, Cotman CW (1978) Biochemical correlates of transmission mediated by glutamate and aspartate. J Neurochem 31:147–155
Plaitakis A, Berl S, Yahr MD (1984) Neurological disorders associated with deficiency of glutamate dehydrogenase. Ann Neurol 15:144–153
Aoki C, Milner TA, Sheu KF, Blass JP, Pickel VM (1987) Regional distribution of astrocytes with intense immunoreactivity for glutamate dehydrogenase in rat brain: implications for neuron-glia interactions in glutamate transmission. J Neurosci 7:2214–2231
Wenthold RJ, Altschuler RA, Skaggs KK, Reeks KA (1987) Immunocytochemical characterization of glutamate dehydrogenase in the cerebellum of the rat. J Neurochem 48:636–643
Kaneko T, Akiyama H, Mizuno N (1987) Immunohistochemical demonstration of glutamate dehydrogenase in astrocytes. Neurosci Lett 77:171–175
Aoki C, Milner TA, Berger SB, Sheu KF, Blass JP, Pickel VM (1987) Glial glutamate dehydrogenase: ultrastructural localization and regional distribution in relation to the mitochondrial enzyme, cytochrome oxidase. J Neurosci Res 18:305–318
Rothe F, Brosz M, Storm-Mathisen J (1994) Quantitative ultrastructural localization of glutamate dehydrogenase in the rat cerebellar cortex. Neuroscience 62:1133–1146
Yu AC, Schousboe A, Hertz L (1982) Metabolic fate of 14 C-labeled glutamate in astrocytes in primary cultures. J Neurochem 39:954–960
Farinelli SE, Nicklas WJ (1992) Glutamate in rat cortical astrocyte cultures. J Neurochem 58:1905–1915
Plaitakis A, Metaxari M, Shashidharan P (2000) Nerve tissue-specific (GLUD2) and housekeeping (GLUD1) human glutamate dehydrogenases are regulated by distinct allosteric mechanisms: implications for biologic function. J Neurochem 75:1862–1869
Mastorodemos V, Zaganas I, Spanaki C, Bessa M, Plaitakis A (2005) Molecular basis of human glutamate dehydrogenase regulation under changing energy demands. J Neurosci Res 79:65–73
McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393
McKenna MC (2013) Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 4:191
Frigerio F, Karaca M, De Roo M, Mlynárik V, Skytt DM, Carobbio S, Pajęcka K, Waagepetersen HS, Gruetter R, Muller D, Maechler P (2012) Deletion of glutamate dehydrogenase 1 (Glud1) in the central nervous system affects glutamate handling without altering synaptic transmission. J Neurochem 123:342–348
Karaca M, Frigerio F, Migrenne S, Martin-Levilain J, Skytt DM, Pajecka K, Martin-del-Rio R, Gruetter R, Tamarit-Rodriguez J, Waagepetersen HS, Magnan C, Maechler P (2015) GDH-dependent glutamate oxidation in the brain dictates peripheral energy substrate distribution. Cell Rep 13:365–375
Nissen JD, Pajęcka K, Stridh MH, Skytt DM, Waagepetersen HS (2015) Dysfunctional TCA-Cycle metabolism in glutamate dehydrogenase deficient astrocytes. Glia 63:2313–2326
Shashidharan P, Michaelidis TM, Robakis NK, Kresovali A, Papamatheakis J, Plaitakis A (1994) Novel human glutamate dehydrogenase expressed in neural and testicular tissues and encoded by an X-linked intronless gene. J Biol Chem 269:16971–16976
Shashidharan P, Clarke DD, Ahmed N, Moschonas N, Plaitakis A (1997) Nerve tissue-specific human glutamate dehydrogenase that is thermolabile and highly regulated by ADP. J Neurochem 68:1804–1811
Burki F, Kaessmann H (2004) Birth and adaptive evolution of a hominoid gene that supports high neurotransmitter flux. Nat Genet 36:1061–1063
Zaganas I, Plaitakis A (2002) Single amino acid substitution (G456A) in the vicinity of the GTP binding domain of human housekeeping glutamate dehydrogenase markedly attenuates GTP inhibition and abolishes the cooperative behavior of the enzyme. J Biol Chem 277:26422–26428
Zaganas I, Spanaki C, Karpusas M, Plaitakis A (2002) Substitution of Ser for Arg-443 in the regulatory domain of human housekeeping (GLUD1) glutamate dehydrogenase virtually abolishes basal activity and markedly alters the activation of the enzyme by ADP and l-leucine. J Biol Chem 277:46552–46558
Kanavouras K, Mastorodemos V, Borompokas N, Spanaki C, Plaitakis A (2007) Properties and molecular evolution of human GLUD2 (neural and testicular tissue-specific) glutamate dehydrogenase. J Neurosci Res 85:3398–3406
Azarias G, Perreten H, Lengacher S, Poburko D, Demaurex N, Magistretti PJ, Chatton JY (2011) Glutamate transport decreases mitochondrial pH and modulates oxidative metabolism in astrocytes. J Neurosci 31:3550–3559
Karim Z, Szutkowska M, Vernimmen C, Bichara M (2005) Renal handling of NH3/NH4+: recent concepts. Nephron Physiol 101:77–81
Chen R, Nishimura MC, Kharbanda S, Peale F, Deng Y, Daemen A, Forrest WF, Kwong M, Hedehus M, Hatzivassiliou G, Friedman LS, Phillips HS (2014) Hominoid-specific enzyme GLUD2 promotes growth of IDH1R132H glioma. Proc Natl Acad Sci USA 111:14217–14222
Mastorodemos V, Kanavouras K, Sundaram S, Providaki M, Petraki Z, Kokkinidis M, Zaganas I, Logothetis DE, Plaitakis A (2015) Side-chain interactions in the regulatory domain of human glutamate dehydrogenase determine basal activity and regulation. J Neurochem 133:73–82
Plaitakis A, Latsoudis H, Spanaki C (2011) The human GLUD2 glutamate dehydrogenase and its regulation in health and disease. Neurochem Int 59:495–509
Spanaki C, Kotzamani D, Petraki Z, Drakos E, Plaitakis A (2014) Heterogeneous cellular distribution of glutamate dehydrogenase in brain and in non-neural tissues. Neurochem Res 39:500–515
Rothe F, Wolf G, Schünzel G (1990) Immunohistochemical demonstration of glutamate dehydrogenase in the postnatally developing rat hippocampal formation and cerebellar cortex: comparison to activity staining. Neuroscience 39:419–429
Zaganas I, Waagepetersen HS, Georgopoulos P, Sonnewald U, Plaitakis A, Schousboe A (2001) Differential expression of glutamate dehydrogenase in cultured neurons and astrocytes from mouse cerebellum and cerebral cortex. J Neurosci Res 66:909–913
Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M (2007) The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci 27:12255–12266
Spanaki C, Zaganas I, Kleopa KA, Plaitakis A (2010) Human GLUD2 glutamate dehydrogenase is expressed in neural and testicular supporting cells. J Biol Chem 285:16748–16756
Spanaki C, Kotzamani D, Petraki Z, Drakos E, Plaitakis A (2015) Expression of human GLUD1 and GLUD2 glutamate dehydrogenases in steroid producing tissues. Mol Cell Endocrinol 415:1–11
Brosnan JT, Brosnan ME, Charron R, Nissim I (1996) A mass isotopomer study of urea and glutamine synthesis from 15 N-labeled ammonia in the perfused rat liver. J Biol Chem 271:16199–16207
Nissim I, Brosnan ME, Yudkoff M, Brosnan JT (1999) Studies of hepatic glutamine metabolism in the perfused rat liver with (15) N-labeled glutamine. J Biol Chem 274:28958–28965
Brosnan ME, Brosnan JT (2009) Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 90:857s–861s
Boon L, Geerts WJ, Jonker A, Lamers WH, Van Noorden CJ (1999) High protein diet induces pericentral glutamate dehydrogenase and ornithine aminotransferase to provide sufficient glutamate for pericentral detoxification of ammonia in rat liver lobules. Histochem Cell Biol 111:445–452
Spanaki C, Plaitakis A (2012) The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox Res 21:117–127
Nissim I (1999) Newer aspects of glutamine/glutamate metabolism: the role of acute pH changes. Am J Physiol 277:493–497
Shapiro RA, Morehouse RF, Curthoys NP (1982) Inhibition by glutamate of phosphate-dependent glutaminase of rat kidney. Biochem J 207:561–566
Borompokas N, Papachatzaki MM, Kanavouras K, Mastorodemos V, Zaganas I, Spanaki C, Plaitakis A (2010) Estrogen modification of human glutamate dehydrogenases is linked to enzyme activation state. J Biol Chem 285:31380–31387
Owen OE, Kalhan SC, Hanson RW (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277:30409–30412
Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, Rutter J, Merritt ME, DeBerardinis RJ (2014) Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell 56:414–424
Spanaki C, Kotzamani D, Kleopa K, Plaitakis A (2015) Evolution of GLUD2 glutamate dehydrogenase allows expression in human cortical neurons. Mol Neurobiol. doi:10.1007/s12035-015-9429-2
Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287
Smith E (1979) The evolution of glutamate dehydrogenases and a hypothesis for the insertion or deletion of multiple residues in the interior of polypeptide chains. Proc Am Philos Soc 123:73–84
Salminen A, Kauppinen A, Hiltunen M, Kaarniranta K (2014) Krebs cycle intermediates regulate DNA and histone methylation: epigenetic impact on the aging process. Ageing Res Rev 16:45–65
Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB (2015) Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518:413–416
Sonnewald U, Westergaard N, Petersen SB, Unsgård G, Schousboe A (1993) Metabolism of [U-13C] glutamate in astrocytes studied by 13 C NMR spectroscopy: incorporation of more label into lactate than into glutamine demonstrates the importance of the tricarboxylic acid cycle. J Neurochem 61:1179–1182
Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC (2014) Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol 11:13–30
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
di Prisco G, Banay-Schwartz M, Strecker HJ (1968) Glutamate dehydrogenase in nuclear and mitochondrial fractions of rat liver. Biochem Biophys Res Commun 33:606–612
Koch-Nolte F, Fischer S, Haag F, Ziegler M (2011) Compartmentation of NAD+-dependent signalling. FEBS Lett 585:1651–1656
Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, Hashimoto K, Zhang N, Flaim E, Michelakis ED (2014) A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 158:84–97
Bao X, Pal R, Hascup KN, Wang Y, Wang WT, Xu W, Hui D, Agbas A, Wang X, Michaelis ML, Choi IY, Belousov AB, Gerhardt GA, Michaelis EK (2009) Transgenic expression of Glud1 (glutamate dehydrogenase 1) in neurons: in vivo model of enhanced glutamate release, altered synaptic plasticity, and selective neuronal vulnerability. J Neurosci 29:13929–13944
Schaller B, Mekle R, Xin L, Kunz N, Gruetter R (2013) Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7T. J Neurosci Res 91:1076–1083
Sonnewald U (2014) Glutamate synthesis has to be matched by its degradation–where do all the carbons go? J Neurochem 131:399–406
Palaiologos G, Hertz L, Schousboe A (1989) Role of aspartate aminotransferase and mitochondrial dicarboxylate transport for release of endogenously and exogenously supplied neurotransmitter in glutamatergic neurons. Neurochem Res 14:359–366
McKenna MC, Stevenson JH, Huang X, Hopkins IB (2000) Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int 37:229–241
Elston GN (2003) Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex 13:1124–1138
Sherwood CC, Lee PW, Rivara CB, Holloway RL, Gilissen EP, Simmons RM, Hakeem A, Allman JM, Erwin JM, Hof PR (2003) Evolution of specialized pyramidal neurons in primate visual and motor cortex. Brain Behav Evol 61:28–44
Cavallaro S, Meiri N, Yi CL, Musco S, Ma W, Goldberg J, Alkon DL (1997) Late memory-related genes in the hippocampus revealed by RNA fingerprinting. Proc Natl Acad Sci USA 94:9669–9673
Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH (2014) Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci USA 111:486–491
Li Q, Guo S, Jiang X, Bryk J, Naumann R, Enard W, Tomita M, Sugimoto M, Khaitovich P, Pääbo S (2016) Mice carrying a human GLUD2 gene recapitulate aspects of human transcriptome and metabolome development. Proc Natl Acad Sci USA 113:5358–5363
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Spanaki, C., Kotzamani, D. & Plaitakis, A. Widening Spectrum of Cellular and Subcellular Expression of Human GLUD1 and GLUD2 Glutamate Dehydrogenases Suggests Novel Functions. Neurochem Res 42, 92–107 (2017). https://doi.org/10.1007/s11064-016-1986-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1986-x