Abstract
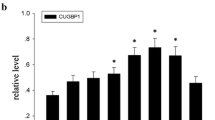
PCBP2, a member of the poly(C)-binding protein (PCBP) family, plays a pivotal role in posttranscriptional and translational regulation by interacting with single-stranded poly(C) motifs in target mRNAs. It is reported that several PCBP family members are involved in human malignancies. However, the distribution and function of PCBP2 in the central nervous system (CNS) remain unclear. In this study, we performed an acute spinal cord injury (SCI) model in adult rats and investigated the dynamic changes of PCBP2 expression in the spinal cord. Western blot and immunohistochemistry analysis revealed that PCBP2 presented in normal spinal cord. It gradually increased, reached a peak at 3 day, and then declined to basal levels at 14 days after SCI. We observed that the expression of PCBP2 was enhanced in the gray and white matter. Immunofluorescence indicated that PCBP2 was located in the neurons and astrocytes. Moreover, colocalization of PCBP2/active caspase-3 was detected in neurons, and colocalization of PCBP2/proliferating cell nuclear antigen was detected in astrocytes after SCI. These results indicated that PCBP2 might play an important role in neuronal apoptosis and astrocyte proliferation. In vitro, PCBP2-specific siRNA-transfected neuron showed significantly decrease of neuronal apoptosis and expression of cell cycle related proteins following glutamate stimulation. Meanwhile, PCBP2 knockdown also reduced primary astrocytes proliferation. All above indicated that PCBP2 might play a crucial role in cell proliferation and apoptosis. Collectively, our data suggested that PCBP2 might play important roles in CNS pathophysiology after SCI.







Similar content being viewed by others
References
Johnson RL, Gabella BA, Gerhart KA, McCray J, Menconi JC, Whiteneck GG (1997) Evaluating sources of traumatic spinal cord injury surveillance data in Colorado. Am J Epidemiol 146:266–272
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24:254–264
Tator CH (1996) Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med 19:206–214
Kan EM, Ling EA, Lu J (2012) Microenvironment changes in mild traumatic brain injury. Brain Res Bull 87:359–372
Becker EB, Bonni A (2004) Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol 72:1–25
Grasso S, Bramanti V, Tomassoni D, Bronzi D, Malfa G, Traini E, Napoli M, Renis M, Amenta F, Avola R (2014) Effect of lipoic acid and alpha-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J Neurosci Res 92:86–94
Bramanti V, Grasso S, Tomassoni D, Traini E, Raciti G, Viola M, Li Volti G, Campisi A, Amenta F, Avola R (2015) Effect of growth factors and steroid hormones on heme oxygenase and cyclin D1 expression in primary astroglial cell cultures. J Neurosci Res 93:521–529
Carnevale D, De Simone R, Minghetti L (2007) Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Disord: Drug Targets 6:388–397
Bramanti V, Tomassoni D, Grasso S, Bronzi D, Napoli M, Campisi A, Li Volti G, Ientile R, Amenta F, Avola R (2012) Cholinergic precursors modulate the expression of heme oxigenase-1, p21 during astroglial cell proliferation and differentiation in culture. Neurochem Res 37:2795–2804
Bramanti V, Tomassoni D, Bronzi D, Grasso S, Curro M, Avitabile M, Li Volsi G, Renis M, Ientile R, Amenta F, Avola R (2010) Alpha-lipoic acid modulates GFAP, vimentin, nestin, cyclin D1 and MAP-kinase expression in astroglial cell cultures. Neurochem Res 35:2070–2077
Chong ZZ, Li F, Maiese K (2006) Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res 3:25–39
Bramanti V, Grasso S, Tibullo D, Giallongo C, Raciti G, Viola M, Avola R (2015) Modulation of extracellular signal-related kinase, cyclin D1, glial fibrillary acidic protein, and vimentin expression in estradiol-pretreated astrocyte cultures treated with competence and progression growth factors. J Neurosci Res 93:1378–1387
Tommerup N, Leffers H (1996) Assignment of human KH-box-containing genes by in situ hybridization: HNRNPK maps to 9q21.32-q21.33, PCBP1 to 2p12-p13, and PCBP2 to 12q13.12-q13.13, distal to FRA12A. Genomics 32:297–298
Sean P, Nguyen JH, Semler BL (2008) The linker domain of poly(rC) binding protein 2 is a major determinant in poliovirus cap-independent translation. Virology 378:243–253
Sean P, Nguyen JH, Semler BL (2009) Altered interactions between stem-loop IV within the 5′ noncoding region of coxsackievirus RNA and poly(rC) binding protein 2: effects on IRES-mediated translation and viral infectivity. Virology 389:45–58
Rieder E, Xiang W, Paul A, Wimmer E (2003) Analysis of the cloverleaf element in a human rhinovirus type 14/poliovirus chimera: correlation of subdomain D structure, ternary protein complex formation and virus replication. J Gen Virol 84:2203–2216
Fukushi S, Okada M, Kageyama T, Hoshino FB, Nagai K, Katayama K (2001) Interaction of poly(rC)-binding protein 2 with the 5′-terminal stem loop of the hepatitis C-virus genome. Virus Res 73:67–79
Tingting P, Caiyun F, Zhigang Y, Pengyuan Y, Zhenghong Y (2006) Subproteomic analysis of the cellular proteins associated with the 3′ untranslated region of the hepatitis C virus genome in human liver cells. Biochem Biophys Res Commun 347:683–691
You F, Sun H, Zhou X, Sun W, Liang S, Zhai Z, Jiang Z (2009) PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat Immunol 10:1300–1308
Roychoudhury P, Paul RR, Chowdhury R, Chaudhuri K (2007) HnRNP E2 is downregulated in human oral cancer cells and the overexpression of hnRNP E2 induces apoptosis. Mol Carcinog 46:198–207
Han W, Xin Z, Zhao Z, Bao W, Lin X, Yin B, Zhao J, Yuan J, Qiang B, Peng X (2013) RNA-binding protein PCBP2 modulates glioma growth by regulating FHL3. J Clin Investig 123:2103–2118
Hu CE, Liu YC, Zhang HD, Huang GJ (2014) The RNA-binding protein PCBP2 facilitates gastric carcinoma growth by targeting miR-34a. Biochem Biophys Res Commun 448:437–442
Morris GF, Mathews MB (1989) Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem 264:13856–13864
Harrop JS, Sharan AD, Przybylski GJ (2000) Epidemiology of spinal cord injury after acute odontoid fractures. Neurosurg Focus 8:e4
McDonald JW, Sadowsky C (2002) Spinal-cord injury. Lancet 359:417–425
Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden AI (2007) Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain 130:2977–2992
Cernak I, Stoica B, Byrnes KR, Di Giovanni S, Faden AI (2005) Role of the cell cycle in the pathobiology of central nervous system trauma. Cell Cycle 4:1286–1293
Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI (2003) Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann Neurol 53:454–468
Tian DS, Yu ZY, Xie MJ, Bu BT, Witte OW, Wang W (2006) Suppression of astroglial scar formation and enhanced axonal regeneration associated with functional recovery in a spinal cord injury rat model by the cell cycle inhibitor olomoucine. J Neurosci Res 84:1053–1063
Du Z, Fenn S, Tjhen R, James TL (2008) Structure of a construct of a human poly(C)-binding protein containing the first and second KH domains reveals insights into its regulatory mechanisms. J Biol Chem 283:28757–28766
Iwanaga K, Sueoka N, Sato A, Hayashi S, Sueoka E (2005) Heterogeneous nuclear ribonucleoprotein B1 protein impairs DNA repair mediated through the inhibition of DNA-dependent protein kinase activity. Biochem Biophys Res Commun 333:888–895
Hay DC, Kemp GD, Dargemont C, Hay RT (2001) Interaction between hnRNPA1 and IkappaBalpha is required for maximal activation of NF-kappaB-dependent transcription. Mol Cell Biol 21:3482–3490
Eversole A, Maizels N (2000) In vitro properties of the conserved mammalian protein hnRNP D suggest a role in telomere maintenance. Mol Cell Biol 20:5425–5432
Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI (2005) Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci USA 102:8333–8338
Wang ZB, Liu YQ, Cui YF (2005) Pathways to caspase activation. Cell Biol Int 29:489–496
Wu J, Stoica BA, Faden AI (2011) Cell cycle activation and spinal cord injury. Neurotherapeutics 8:221–228
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81171140, 81471258), the Colleges and Universities in Natural Science Research Project of Jiangsu Province (No. 13KJB31009), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Xingxing Mao and Jin Liu are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mao, X., Liu, J., Chen, C. et al. PCBP2 Modulates Neural Apoptosis and Astrocyte Proliferation After Spinal Cord Injury. Neurochem Res 41, 2401–2414 (2016). https://doi.org/10.1007/s11064-016-1953-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1953-6