Abstract
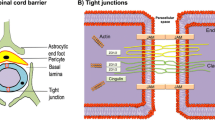
Spinal cord injury (SCI) induces the disruption of the blood-spinal cord barrier (BSCB), which leads to infiltration of blood cells, inflammatory responses and neuronal cell death, with subsequent development of spinal cord secondary damage. Recent reports pointed to an important role of retinoic acid (RA), the active metabolite of the vitamin A, in the induction of the blood–brain barrier (BBB) during human and mouse development, however, it is unknown whether RA plays a role in maintaining BSCB integrity under the pathological conditions such as SCI. In this study, we investigated the BSCB protective role of RA both in vivo and in vitro and demonstrated that autophagy are involved in the BSCB protective effect of RA. Our data show that RA attenuated BSCB permeability and also attenuated the loss of tight junction molecules such as P120, β-catenin, Occludin and Claudin5 after injury in vivo as well as in brain microvascular endothelial cells. In addition, RA administration improved functional recovery of the rat model of trauma. We also found that RA could significantly increase the expression of LC3-II and decrease the expression of p62 both in vivo and in vitro. Furthermore, combining RA with the autophagy inhibitor chloroquine (CQ) partially abolished its protective effect on the BSCB and exacerbated the loss of tight junctions. Together, our studies indicate that RA improved functional recovery in part by the prevention of BSCB disruption via the activation of autophagic flux after SCI.






Similar content being viewed by others
Change history
14 October 2020
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11064-020-03149-1.
References
Mizee MR, de Vries HE (2013) Blood-brain barrier regulation. Tissue Barriers 1(5):26881–26886. doi:10.4161/tisb.26882/JNEUROSCI.1338-12.2013
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37(1):13–25. doi:10.1016/j.nbd.2009.07.030
Cardoso FL, Brites D, Brito MA (2010) Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 64(2):328–363. doi:10.1016/j.brainresrev.2010.05.003
Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M (2011) The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 70(2):194–206. doi:10.1002/ana.22421
Lee JY, Kim HS, Choi HY, Oh TH, Yune TY (2012) Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain J Neurol 135(Pt 8):2375–2389. doi:10.1093/brain/aws171
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12(9):814–822. doi:10.1038/ncb0910-814
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. doi:10.1016/j.cell.2011.10.026
Ribas VT, Schnepf B, Challagundla M, Koch JC, Bahr M, Lingor P (2015) Early and sustained activation of autophagy in degenerating axons after spinal cord injury. Brain Pathol 25(2):157–170. doi:10.1111/bpa.12170
Qin Z (2014) Changes in autophagy proteins in a rat model of spinal cord injury. Chin J Traumatol 17(4):193–197. doi:10.3760/cma.j.issn.1008-1275.2014.04.002
Park Y, Liu C, Luo T, Bramlett H, Dietrich WD, Hu B (2015) Chaperone-mediated autophagy after traumatic brain injury. J Neurotrauma. doi:10.1089/neu.2014.3694
Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L (2014) Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol 49(1):276–287. doi:10.1007/s12035-013-8518-3
Smith CM, Mayer JA, Duncan ID (2013) Autophagy promotes oligodendrocyte survival and function following dysmyelination in a long-lived myelin mutant. J Neurosci Off J Soc Neurosci 33(18):8088–8100. doi:10.1523/JNEUROSCI.0233-13.2013
Chang CP, Su YC, Hu CW, Lei HY (2013) TLR2-dependent selective autophagy regulates NF-kappaB lysosomal degradation in hepatoma-derived M2 macrophage differentiation. Cell Death Differ 20(3):515–523. doi:10.1038/cdd.2012.146
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E (2011) Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine 36(22):E1427–E1434. doi:10.1097/BRS.0b013e3182028c3a
Niederreither K, Dolle P (2008) Retinoic acid in development: towards an integrated view. Nat Rev Genet 9(7):541–553. doi:10.1038/nrg2340
Kornyei Z, Gocza E, Ruhl R, Orsolits B, Voros E, Szabo B, Vagovits B, Madarasz E (2007) Astroglia-derived retinoic acid is a key factor in glia-induced neurogenesis. FASEB J 21(10):2496–2509. doi:10.1096/fj.06-7756com
Shearer KD, Fragoso YD, Clagett-Dame M, McCaffery PJ (2012) Astrocytes as a regulated source of retinoic acid for the brain. Glia 60(12):1964–1976. doi:10.1002/glia.22412
Paschaki M, Lin SC, Wong RL, Finnell RH, Dolle P, Niederreither K (2012) Retinoic acid-dependent signaling pathways and lineage events in the developing mouse spinal cord. PLoS One 7(3):e32447. doi:10.1371/journal.pone.0032447
Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, de Vries HE, Reijerkerk A (2013) Retinoic acid induces blood-brain barrier development. J Neurosci 33(4):1660–1671. doi:10.1523/JNEUROSCI.1338-12.2013
Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV (2014) A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep 4:4160. doi:10.1038/srep04160
Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, Wang G, Chen Z (2013) Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9(9):1321–1333. doi:10.4161/auto.25132
Zhang X, Yuan Y, Jiang L, Zhang J, Gao J, Shen Z, Zheng Y, Deng T, Yan H, Li W, Hou WW, Lu J, Shen Y, Dai H, Hu WW, Zhang Z, Chen Z (2014) Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: involvement of PARK2-dependent mitophagy. Autophagy 10(10):1801–1813. doi:10.4161/auto.32136
Zhang H, Wu F, Kong X, Yang J, Chen H, Deng L, Cheng Y, Ye L, Zhu S, Zhang X, Wang Z, Shi H, Fu X, Li X, Xu H, Lin L, Xiao J (2014) Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J Transl Med 12:130. doi:10.1186/1479-5876-12-130
van Neerven S, Mey J, Joosten EA, Steinbusch HW, van Kleef M, Marcus MA, Deumens R (2010) Systemic but not local administration of retinoic acid reduces early transcript levels of pro-inflammatory cytokines after experimental spinal cord injury. Neurosci Lett 485(1):21–25. doi:10.1016/j.neulet.2010.08.051
Wang HL, Lai TW (2014) Optimization of Evans blue quantitation in limited rat tissue samples. Sci Rep 4:6588. doi:10.1038/srep06588
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201. doi:10.1016/j.neuron.2008.01.003
Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY (2012) Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem 121(5):818–829. doi:10.1111/j.1471-4159.2012.07731.x
Fassbender JM, Saraswat-Ohri S (2012) Deletion of endoplasmic reticulum stress-induced CHOP protects microvasculature post-spinal cord injury. Curr Neurovasc Res 9(1875–5739):274–281
Pun PB, Lu J, Moochhala S (2009) Involvement of ROS in BBB dysfunction. Free Radic Res 43(4):348–364. doi:10.1080/10715760902751902
Olmez I, Ozyurt H (2012) Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int 60(2):208–212. doi:10.1016/j.neuint.2011.11.009
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584–1596. doi:10.1038/nm.3407
Li XQ, Lv HW (2014) Role of the TLR4 pathway in blood spinal cord barrier dysfunction during the bimodal stage after ischemiareperfusion injury in rats. J Neuroinflammation 2014(7):28–42
Xiao-Qian Li JW (2014) Intrathecal antagonism of microglial TLR4 reduces inflammatory damage to blood–spinal cord barrier following ischemia/reperf usion injury in rats. Mol Brain 2014(7):28
Xanthos DN, Pungel I, Wunderbaldinger G, Sandkuhler J (2012) Effects of peripheral inflammation on the blood-spinal cord barrier. Mol Pain 8:44. doi:10.1186/1744-8069-8-44
Fan ZK, Lv G, Wang YF, Li G, Yu DS, Wang YS, Zhang YQ, Mei XF, Cao Y (2013) The protective effect of salvianolic acid B on blood-spinal cord barrier after compression spinal cord injury in rats. J Mol Neurosc 51(3):986–993. doi:10.1007/s12031-013-0083-8
Rajawat Y, Hilioti Z, Bossis I (2010) Autophagy A target for retinoic acids. Autophagy 6(8):1224–1226. doi:10.4161/auto.6.8.13793/ars.2010.3491
Niapour N, Niapour A, Sheikhkanloui Milan H, Amani M, Salehi H, Najafzadeh N, Gholami MR (2015) All trans retinoic acid modulates peripheral nerve fibroblasts viability and apoptosis. Tissue Cell 47(1):61–65. doi:10.1016/j.tice.2014.11.004
Wang Y, He PC, Qi J, Liu YF, Zhang M (2015) Tetra-arsenic tetra-sulfide induces cell cycle arrest and apoptosis in retinoic acid-resistant acute promyelocytic leukemia cells. Biomed Rep 3(4):583–587. doi:10.3892/br.2015.466
Liang C, Yang L, Guo S (2015) All- retinoic acid inhibits migration, invasion and proliferation, and promotes apoptosis in glioma cells. Oncol Lett 9(6):2833–2838. doi:10.3892/ol.2015.3120
Schrage K, Koopmans G, Joosten EA, Mey J (2006) Macrophages and neurons are targets of retinoic acid signaling after spinal cord contusion injury. Eur J Neurosci 23(2):285–295. doi:10.1111/j.1460-9568.2005.04534.x
Mey J, Morassutti DJ, Brook G, Liu RH, Zhang YP, Koopmans G, McCaffery P (2005) Retinoic acid synthesis by a population of NG2-positive cells in the injured spinal cord. Eur J Neurosci 21(6):1555–1568. doi:10.1111/j.1460-9568.2005.03928.x
Yip PK, Wong LF, Pattinson D, Battaglia A, Grist J, Bradbury EJ, Maden M, McMahon SB, Mazarakis ND (2006) Lentiviral vector expressing retinoic acid receptor beta2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord. Hum Mol Genet 15(21):3107–3118. doi:10.1093/hmg/ddl251
Mizee MR, Nijland PG, van der Pol SM, Drexhage JA, van Het Hof B, Mebius R, van der Valk P, van Horssen J, Reijerkerk A, de Vries HE (2014) Astrocyte-derived retinoic acid: a novel regulator of blood-brain barrier function in multiple sclerosis. Acta Neuropathol 128(5):691–703. doi:10.1007/s00401-014-1335-6
Alirezaei M, Kemball CC, Whitton JL (2011) Autophagy, inflammation and neurodegenerative disease. Eur J Neurosci 33(2):197–204. doi:10.1111/j.1460-9568.2010.07500.x
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29(9):528–535. doi:10.1016/j.tins.2006.07.003
Xie Y, You SJ, Zhang YL, Han Q, Cao YJ, Xu XS, Yang YP, Li J, Liu CF (2011) Protective role of autophagy in AGE-induced early injury of human vascular endothelial cells. Mol Med Rep 4(3):459–464. doi:10.3892/mmr.2011.460
Li H, Gao A, Feng D, Wang Y, Zhang L, Cui Y, Li B, Wang Z, Chen G (2014) Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood-brain barrier integrity during experimental cerebral ischemia-reperfusion injury. Transl Stroke Res 5(5):618–626. doi:10.1007/s12975-014-0354-x
Nighot PK, Hu CA, Ma TY (2015) Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem 290(11):7234–7246. doi:10.1074/jbc.M114.597492
Zhao H, Ji Z, Tang D, Yan C, Zhao W, Gao C (2013) Role of autophagy in early brain injury after subarachnoid hemorrhage in rats. Mol Biol Rep 40(2):819–827. doi:10.1007/s11033-012-2120-z
van Vliet EA, Forte G, Holtman L, den Burger JC, Sinjewel A, de Vries HE, Aronica E, Gorter JA (2012) Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia 53(7):1254–1263. doi:10.1111/j.1528-1167.2012.03513.x
Yu F, Wang Z, Tanaka M, Chiu CT, Leeds P, Zhang Y, Chuang DM (2013) Posttrauma cotreatment with lithium and valproate: reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury. J Neurosurg 119(3):766–773. doi:10.3171/2013.6.JNS13135
Zeng M, Zhou JN (2008) Roles of autophagy and mTOR signaling in neuronal differentiation of mouse neuroblastoma cells. Cell Signal 20(4):659–665. doi:10.1016/j.cellsig.2007.11.015
Trocoli A, Mathieu J, Priault M, Reiffers J, Souquere S, Pierron G, Besancon F, Djavaheri-Mergny M (2011) ATRA-induced upregulation of Beclin 1 prolongs the life span of differentiated acute promyelocytic leukemia cells. Autophagy 7(10):1108–1114. doi:10.4161/auto.7.10.16623
Acknowledgments
This study was partially supported by a research Grant from the National Natural Science Funding of China (81302775, 81472165, 81200958, 81372112), Zhejiang Provincial Natural Science Foundation of China (LY14H090013, LY14H150010, LY14H170002), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (to J.X.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s41980-020-00472-9
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2015_1756_MOESM1_ESM.tif
RA inhibits apoptosis protein caspase-12 expression after SCI. Representative western blots and quantification data of Cle-caspase-12 in each group rats. *P < 0.01, versus SCI group. Data represent mean values ± SEM, n = 4 (TIFF 347 kb)
About this article
Cite this article
Zhou, Y., Zheng, B., Ye, L. et al. RETRACTED ARTICLE: Retinoic Acid Prevents Disruption of Blood-Spinal Cord Barrier by Inducing Autophagic Flux After Spinal Cord Injury. Neurochem Res 41, 813–825 (2016). https://doi.org/10.1007/s11064-015-1756-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1756-1