Abstract
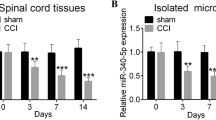
Chronic neuropathic pain is an unfavourable pathological pain characterised by allodynia and hyperalgesia which has brought considerable trouble to people’s physical and mental health, but effective therapeutics are still lacking. MicroRNAs (miRNAs) have been widely studied in the development of neuropathic pain and neuronal inflammation. Among various miRNAs, miR-155 has been widely studied. It is intensively involved in regulating inflammation-associated diseases. However, the role of miR-155 in regulating neuropathic pain development is poorly understood. In the present study, we aimed to investigate whether miR-155 is associated with neuropathic pain and delineate the underlying mechanism. Using a neuropathic pain model of chronic constriction injury (CCI), miR-155 expression levels were markedly increased in the spinal cord. Inhibition of miR-155 significantly attenuated mechanical allodynia, thermal hyperalgesia and proinflammatory cytokine expression. We also demonstrated that miR-155 directly bound with the 3′-untranslated region of the suppressor of cytokine signalling 1 (SOCS1). The expression of SOCS1 significantly decreased in the CCI rat model, but this effect could be reversed by miR-155 inhibition. Furthermore, knockdown of SOCS1 abrogated the inhibitory effects of miR-155 inhibition on neuropathic development and neuronal inflammation. Finally, we demonstrated that inhibition of miR-155 resulted in the suppression of nuclear factor-κB and p38 mitogen-activated protein kinase activation by mediating SOCS1. Our data demonstrate the critical role of miR-155 in regulating neuropathic pain through SOCS1, and suggest that miR-155 may be an important and potential target in preventing neuropathic pain development.






Similar content being viewed by others
Abbreviations
- MiRNAs:
-
MicroRNAs
- CCI:
-
Chronic constriction injury
- SOCS1:
-
Suppressor of cytokine signalling 1
- NF-κB:
-
Nuclear factor-κB
- p38 MAPK:
-
p38 mitogen-activated protein kinase
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- 3′-UTR:
-
3′-Untranslated region
References
Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan M, Herbert TA, Yarkoni-Abitbul M, Tichauer D, Livneh J, Gershon E, Zheng M, Tan K, John SL, Slade GD, Jordan J, Woolf CJ, Peltz G, Maixner W, Diatchenko L, Seltzer Z, Salter MW, Mogil JS (2012) Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med 18:595–599
Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS (2007) Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 132:237–251
Finnerup NB, Sindrup SH, Jensen TS (2010) The evidence for pharmacological treatment of neuropathic pain. Pain 150:573–581
Neville A, Peleg R, Singer Y, Sherf M, Shvartzman P (2008) Chronic pain: a population-based study. Isr Med Assoc J 10:676–680
Genevay S, Finckh A, Payer M, Mezin F, Tessitore E, Gabay C, Guerne PA (2008) Elevated levels of tumor necrosis factor-alpha in periradicular fat tissue in patients with radiculopathy from herniated disc. Spine (Phila Pa 1976) 33:2041–2046
McCarron RF, Wimpee MW, Hudkins PG, Laros GS (1987) The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine (Phila Pa 1976) 12:760–764
Vallejo R, Tilley DM, Vogel L, Benyamin R (2010) The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract 10:167–184
Moalem G, Tracey DJ (2006) Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 51:240–264
Ma W, Bisby MA (1998) Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res 797:243–254
Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM, Yao SL (2006) Alleviation of neuropathic pain by intrathecal injection of antisense oligonucleotides to p65 subunit of NF-kappaB. Br J Anaesth 97:553–558
Ledeboer A, Gamanos M, Lai W, Martin D, Maier SF, Watkins LR, Quan N (2005) Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. Eur J Neurosci 22:1977–1986
Schafers M, Svensson CI, Sommer C, Sorkin LS (2003) Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 23:2517–2521
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Xu L, Huang Y, Yu X, Yue J, Yang N, Zuo P (2007) The influence of p38 mitogen-activated protein kinase inhibitor on synthesis of inflammatory cytokine tumor necrosis factor alpha in spinal cord of rats with chronic constriction injury. Anesth Analg 105:1838–1844 (Table of contents)
Meotti FC, Posser T, Missau FC, Pizzolatti MG, Leal RB, Santos AR (2007) Involvement of p38MAPK on the antinociceptive action of myricitrin in mice. Biochem Pharmacol 74:924–931
Sakai A, Suzuki H (2014) Emerging roles of microRNAs in chronic pain. Neurochem Int 77:58–67
Mendell JT, Olson EN (2012) MicroRNAs in stress signaling and human disease. Cell 148:1172–1187
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11:228–234
Imai S, Saeki M, Yanase M, Horiuchi H, Abe M, Narita M, Kuzumaki N, Suzuki T (2011) Change in microRNAs associated with neuronal adaptive responses in the nucleus accumbens under neuropathic pain. J Neurosci 31:15294–15299
Kosik KS (2006) The neuronal microRNA system. Nat Rev Neurosci 7:911–920
Tarassishin L, Loudig O, Bauman A, Shafit-Zagardo B, Suh HS, Lee SC (2011) Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR-155 and miR-155*. Glia 59:1911–1922
Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T (2009) Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience 164:711–723
Chen HP, Zhou W, Kang LM, Yan H, Zhang L, Xu BH, Cai WH (2014) Intrathecal miR-96 inhibits Nav1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem Res 39:76–83
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z, Ding J, Jia L, Yuan W (2013) Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 61:504–512
Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179:5082–5089
Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC (2012) MiR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 135:73–88
Krebs DL, Hilton DJ (2001) SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19:378–387
Yasukawa H, Sasaki A, Yoshimura A (2000) Negative regulation of cytokine signaling pathways. Annu Rev Immunol 18:143–164
He Y, Zhang W, Zhang R, Zhang H, Min W (2006) SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. J Biol Chem 281:5559–5566
Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A (2003) Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity 19:437–450
Chinen T, Kobayashi T, Ogata H, Takaesu G, Takaki H, Hashimoto M, Yagita H, Nawata H, Yoshimura A (2006) Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFNgamma and IL-4 are involved. Gastroenterology 130:373–388
Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW II, Kelley KW, Heijnen CJ, Kavelaars A (2010) Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain 150:550–560
Yaksh TL, Rudy TA (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ (2007) Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci 27:12067–12077
Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE (2008) Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol 213:257–267
Lin X, You Y, Wang J, Qin Y, Huang P, Yang F (2014) MicroRNA-155 deficiency promotes nephrin acetylation and attenuates renal damage in hyperglycemia-induced nephropathy. Inflammation. doi:10.1007/s10753-014-9961-7
Bluml S, Bonelli M, Niederreiter B, Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J, Redlich K (2011) Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum 63:1281–1288
Jin HM, Kim TJ, Choi JH, Kim MJ, Cho YN, Nam KI, Kee SJ, Moon JB, Choi SY, Park DJ, Lee SS, Park YW (2014) MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis. Arthritis Res Ther 16:R88
Singh UP, Murphy AE, Enos RT, Shamran HA, Singh NP, Guan H, Hegde VL, Fan D, Price RL, Taub DD, Mishra MK, Nagarkatti M, Nagarkatti PS (2014) MiR-155 deficiency protects mice from experimental colitis by reducing Th1/Th17 responses. Immunology 143:478–489
Austin PJ, Moalem-Taylor G (2010) The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol 229:26–50
Stein C, Machelska H (2011) Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol Rev 63:860–881
Tiwari V, Guan Y, Raja SN (2014) Modulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveats. Neurosci Biobehav Rev 45C:19–27
Rao R, Nagarkatti P, Nagarkatti M (2014) Staphylococcal enterotoxin B-induced microRNA-155 targets SOCS1 to promote acute inflammatory lung injury. Infect Immun 82:2971–2979
Kim JH, Jou I, Joe EH (2014) Suppression of miR-155 expression in IFN-gamma-treated astrocytes and microglia by DJ-1: a possible mechanism for maintaining SOCS1 expression. Exp Neurobiol 23:148–154
Li X, Tian F, Wang F (2013) Rheumatoid arthritis-associated microRNA-155 targets SOCS1 and upregulates TNF-alpha and IL-1beta in PBMCs. Int J Mol Sci 14:23910–23921
Huang C, Li H, Wu W, Jiang T, Qiu Z (2013) Regulation of miR-155 affects pancreatic cancer cell invasiveness and migration by modulating the STAT3 signaling pathway through SOCS1. Oncol Rep 30:1223–1230
Zhao XD, Zhang W, Liang HJ, Ji WY (2013) Overexpression of miR-155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. PLoS One 8:e56395
Recio C, Oguiza A, Lazaro I, Mallavia B, Egido J, Gomez-Guerrero C (2014) Suppressor of cytokine signaling 1-derived peptide inhibits janus kinase/signal transducers and activators of transcription pathway and improves inflammation and atherosclerosis in diabetic mice. Arterioscler Thromb Vasc Biol 34:1953–1960
Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M (2008) JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem 107:50–60
Muratsu D, Yoshiga D, Taketomi T, Onimura T, Seki Y, Matsumoto A, Nakamura S (2013) Zoledronic acid enhances lipopolysaccharide-stimulated proinflammatory reactions through controlled expression of SOCS1 in macrophages. PLoS One 8:e67906
Dragone T, Cianciulli A, Calvello R, Porro C, Trotta T, Panaro MA (2014) Resveratrol counteracts lipopolysaccharide-mediated microglial inflammation by modulating a SOCS-1 dependent signaling pathway. Toxicol In Vitro 28:1126–1135
Andersen HH, Duroux M, Gazerani P (2014) MicroRNAs as modulators and biomarkers of inflammatory and neuropathic pain conditions. Neurobiol Dis 71:159–168
Li H, Huang Y, Ma C, Yu X, Zhang Z, Shen L (2014) MiR-203 involves in neuropathic pain development and represses Rap1a expression in nerve growth factor differentiated neuronal PC12 cells. Clin J Pain 20:20
Sakai A, Suzuki H (2013) Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem Biophys Res Commun 435:176–181
Nadorp B, Soreq H (2014) Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Front Mol Neurosci 7:9
Kress M, Huttenhofer A, Landry M, Kuner R, Favereaux A, Greenberg D, Bednarik J, Heppenstall P, Kronenberg F, Malcangio M, Rittner H, Uceyler N, Trajanoski Z, Mouritzen P, Birklein F, Sommer C, Soreq H (2013) MicroRNAs in nociceptive circuits as predictors of future clinical applications. Front Mol Neurosci 6:00033
Qi L, Zhang Y (2014) The microRNA 132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through mTOR signaling pathway. Cell Physiol Biochem 33:433–445
Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM (2009) MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol 23:1876–1884
Arai M, Genda Y, Ishikawa M, Shunsuke T, Okabe T, Sakamoto A (2013) The miRNA and mRNA changes in rat hippocampi after chronic constriction injury. Pain Med 14:720–729
David BT, Sampath S, Dong W, Heiman A, Rella CE, Elkabes S, Heary RF (2014) A toll-like receptor 9 antagonist improves bladder function and white matter sparing in spinal cord injury. J Neurotrauma 31:1800–1806
Zhang L, Huang D, Wang Q, Shen D, Wang Y, Chen B, Zhang J, Gai L (2014) MiR-132 inhibits expression of SIRT1 and induces pro-inflammatory processes of vascular endothelial inflammation through blockade of the SREBP-1c metabolic pathway. Cardiovasc Drugs Ther 28:303–311
Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, Adam M, Nagakami F, Heymann HM, Chernugobova E, Jin H, Roy J, Hultgren R, Caidahl K, Schrepfer S, Hamsten A, Eriksson P, McConnell MV, Dalman RL, Tsao PS (2014) MiR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun 5:5214
Wang HJ, Huang YL, Shih YY, Wu HY, Peng CT, Lo WY (2014) MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediators Inflamm 379537:14
Conflict of interests
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, Y., Yang, J., Xiang, K. et al. Suppression of MicroRNA-155 Attenuates Neuropathic Pain by Regulating SOCS1 Signalling Pathway. Neurochem Res 40, 550–560 (2015). https://doi.org/10.1007/s11064-014-1500-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1500-2