Abstract
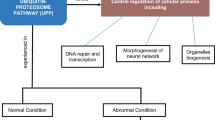
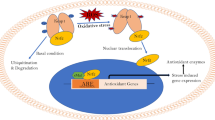
The predominant molecular symptom of aging is the accumulation of altered gene products. Moreover, several conditions including protein, lipid or glucose oxidation disrupt redox homeostasis and lead to accumulation of unfolded or misfolded proteins in the aging brain. Alzheimer’s and Parkinson’s diseases or Friedreich ataxia are neurological diseases sharing, as a common denominator, production of abnormal proteins, mitochondrial dysfunction and oxidative stress, which contribute to the pathogenesis of these so called “protein conformational diseases”. The central nervous system has evolved the conserved mechanism of unfolded protein response to cope with the accumulation of misfolded proteins. As one of the main intracellular redox systems involved in neuroprotection, the vitagene system is emerging as a neurohormetic potential target for novel cytoprotective interventions. Vitagenes encode for cytoprotective heat shock proteins (Hsp) Hsp70 and heme oxygenase-1, as well as thioredoxin reductase and sirtuins. Nutritional studies show that ageing in animals can be significantly influenced by dietary restriction. Thus, the impact of dietary factors on health and longevity is an increasingly appreciated area of research. Reducing energy intake by controlled caloric restriction or intermittent fasting increases lifespan and protects various tissues against disease. Genetics has revealed that ageing may be controlled by changes in intracellular NAD/NADH ratio regulating sirtuin, a group of proteins linked to aging, metabolism and stress tolerance in several organisms. Recent findings suggest that several phytochemicals exhibit biphasic dose responses on cells with low doses activating signaling pathways that result in increased expression of vitagenes encoding survival proteins, as in the case of the Keap1/Nrf2/ARE pathway activated by curcumin and NAD/NADH-sirtuin-1 activated by resveratrol. Consistently, the neuroprotective roles of dietary antioxidants including curcumin, acetyl-l-carnitine and carnosine have been demonstrated through the activation of these redox-sensitive intracellular pathways. Although the notion that stress proteins are neuroprotective is broadly accepted, still much work needs to be done in order to associate neuroprotection with specific pattern of stress responses. In this review the importance of vitagenes in the cellular stress response and the potential use of dietary antioxidants in the prevention and treatment of neurodegenerative disorders is discussed.








Similar content being viewed by others
References
Halliwell B (2007) Oxidative stress and cancer: have we moved forward? Biochem J 401:1–11
Calabrese V, Scapagnini G, Colombrita C, Ravagna A, Pennisi G, Giuffrida Stella AM, Galli F, Butterfield DA (2003) Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids 25:437–444
Poon HF, Calabrese V, Scapagnini G, Butterfield DA (2004) Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontology 59:478–493
Forman HJ, Fukuto JM, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287:246–256
Poon HF, Calabrese V, Scapagnini G, Butterfield DA (2004) Free radicals and brain aging. Clin Geriatr Med 20:329–359
Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM (2004) Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Age Dev 125:325–335
Calabrese V, Giuffrida Stella AM, Butterfield DA, Scapagnini G (2004) Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal 6:895–913
Halliwell B (2002) Hypothesis: proteasomal dysfunction: a primary event in neurogeneration that leads to nitrative and oxidative stress and subsequent cell death. Ann NY Acad Sci 962:182–194
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10:1611–1626
Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, Bozner P (1998) Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol 152:871–877
Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, Tabaton M, Perry G (1998) Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem 70:2212–2215
Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med 7:548–554
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Biol Med 32:1050–1060
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430:631–639
Drew B, Leeuwenburgh C (2002) Aging and the role of reactive nitrogen species. Ann NY Acad Sci 959:66–81
Kroncke KD (2003) Nitrosative stress and transcription. Biol Chem 384:1365–1377
Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA (2004) The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem 385:1–10
Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield DA, Stella AG (2007) Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res 32:757–773
Calabrese V, Boyd-Kimball D, Scapagnini G, Butterfield DA (2004) Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders: the role of vitagenes. In Vivo 18:245–267
Mancuso C, Scapagnini G, Curro D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V (2007) Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci 12:1107–1123
Vina J, Borras C, Gomez-Cabrera MC, Orr WC (2006) Part of the series: from dietary antioxidants to regulators in cellular signalling and gene expression. Role of reactive oxygen species and (phyto)oestrogens in the modulation of adaptive response to stress. Free Radic Res 40:111–119
McCord JM, Fridovich I (1988) Superoxide dismutase: the first twenty years (1968–1988). Free Radic Biol Med 5:363–369
Calabrese EJ, Staudenmayer JW, Stanek EJ (2006) Drug development and hormesis: changing conceptual understanding of the dose response creates new challenges and opportunities for more effective drugs. Curr Opin Drug Discov Devel 9:117–123
Zhang K, Kaufman RJ (2006) The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66:102–109
Calabrese V, Butterfield DA, Scapagnini G, Stella AM, Maines MD (2006) Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal 8:444–477
Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37:517–554
Mancuso C (2004) Heme oxygenase and its products in the nervous system. Antioxid Redox Signal 6:878–887
Stocker R (2004) Antioxidant activities of bile pigments. Antioxid Redox Signal 6:841–849
Mancuso C, Pani G, Calabrese V (2006) Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep 11:207–213
Mancuso C, Bonsignore A, Capone C, Di Stasio E, Pani G (2006) Albumin-bound bilirubin interacts with nitric oxide by a redox mechanism. Antioxid Redox Signal 8:487–494
Mancuso C, Bonsignore A, Di Stasio E, Mordente A, Motterlini R (2003) Bilirubin and S-nitrosothiols interaction: evidence for a possible role of bilirubin as a scavenger of nitric oxide. Biochem Pharmacol 66:2355–2363
Simonian NA, Coyle JT (1996) Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 36:83–106
Sayre LM, Smith MA, Perry G (2001) Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem 8:721–738
Andersen JK (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Med 10:18–25
Mancuso C, Perluigi M, Cini C, De Marco C, Giuffrida Stella AM, Calabrese V (2006) Heme oxygenase and cyclooxygenase in the central nervous system: a functional interplay. J Neurosci Res 84:1385–1391
Panahian N, Yoshiura M, Maines MD (1999) Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem 72:1187–1203
Takeda A, Perry G, Abraham NG, Dwyer BE, Kutty RK, Laitinen JT, Petersen RB, Smith MA (2000) Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J Biol Chem 275:5395–5399
Premkumar DR, Smith MA, Richey PL, Petersen RB, Castellani R, Kutty RK, Wiggert B, Perry G, Kalaria RN (1995) Induction of heme oxygenase-1 mRNA and protein in neocortex and cerebral vessels in Alzheimer’s disease. J Neurochem 65:1399–1402
Schipper HM (2000) Heme oxygenase-1: role in brain aging and neurodegeneration. Exp Gerontol 35:821–830
Mancuso C, Bates TE, Butterfield DA, Calafato S, Cornelius C, De Lorenzo A, Dinkova Kostova AT, Calabrese V (2007) Natural antioxidants in Alzheimer’s disease. Expert Opin Investig Drugs 16:1921–1931
Butterfield D, Castegna A, Pocernich C, Drake J, Scapagnini G, Calabrese V (2002) Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem 13:444–461
Scapagnini G, Colombrita C, Amadio M, D’Agata V, Arcelli E, Sapienza M, Quattrone A, Calabrese V (2006) Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal 8:395–403
Ganguli M, Chandra V, Kamboh MI, Johnston JM, Dodge HH, Thelma BK, Juyal RC, Pandav R, Belle SH, DeKosky ST (2000) Apolipoprotein E polymorphism and Alzheimer disease: The Indo-US Cross-National Dementia Study. Arch Neurol 57:824–830
Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM (2001) The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 21:8370–8377
Wu L, Wang R (2005) Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57:585–630
Kostoglou-Athanassiou I, Forsling ML, Navarra P, Grossman AB (1996) Oxytocin release is inhibited by the generation of carbon monoxide from the rat hypothalamus—further evidence for carbon monoxide as a neuromodulator. Brain Res Mol Brain Res 42:301–306
Mancuso C, Kostoglou-Athanassiou I, Forsling ML, Grossman AB, Preziosi P, Navarra P, Minotti G (1997) Activation of heme oxygenase and consequent carbon monoxide formation inhibits the release of arginine vasopressin from rat hypothalamic explants, molecular linkage between heme catabolism and neuroendocrine function. Brain Res Mol Brain Res 50:267–276
Mancuso C, Ragazzoni E, Tringali G, Liberale I, Preziosi P, Grossman A, Navarra P (1999) Inhibition of heme oxygenase in the central nervous system potentiates endotoxin-induced vasopressin release in the rat. J Neuroimmunol 99:189–194
Parkes D, Kasckow J, Vale W (1994) Carbon monoxide modulates secretion of corticotropin-releasing factor from rat hypothalamic cell cultures. Brain Res 646:315–318
Pozzoli G, Mancuso C, Mirtella A, Preziosi P, Grossman AB, Navarra P (1994) Carbon monoxide as a novel neuroendocrine modulator: inhibition of stimulated corticotropin-releasing hormone release from acute rat hypothalamic explants. Endocrinology 135:2314–2317
Mancuso C, Pistritto G, Tringali G, Grossman AB, Preziosi P, Navarra P (1997) Evidence that carbon monoxide stimulates prostaglandin endoperoxide synthase activity in rat hypothalamic explants and in primary cultures of rat hypothalamic astrocytes. Brain Res Mol Brain Res 45:294–300
Mancuso C, Tringali G, Grossman A, Preziosi P, Navarra P (1998) The generation of nitric oxide and carbon monoxide produces opposite effects on the release of immunoreactive interleukin-1beta from the rat hypothalamus in vitro: evidence for the involvement of different signaling pathways. Endocrinology 139:1031–1037
Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X (2002) Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res 91:610–617
Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell R, Choi AM (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6:422–428
Ryter SW, Otterbein LE, Morse D, Choi AM (2002) Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234–235:249–263
Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK (1999) The neuroprotective potential of heat shock protein 70 (HSP70). Mol Med Today 5:525–531
Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, Yenari MA, Steinberg GK (2002) Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol 52:160–167
Narasimhan P, Swanson RA, Sagar SM, Sharp FR (1996) Astrocyte survival and HSP70 heat shock protein induction following heatshock and acidosis. Glia 17:147–159
Fink SL, Chang LK, Ho DY, Sapolsky RM (1997) Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J Neurochem 68:961–969
Hata R, Maeda K, Hermann D, Mies G, Hossmann KA (2000) Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 20:306–315
Perez N, Sugar J, Charya S, Johnson G, Merril C, Bierer L, Perl D, Haroutunian V, Wallace W (1991) Increased synthesis and accumulation of heat shock 70 proteins in Alzheimer’s disease. Brain Res Mol Brain Res 11:249–254
Yoo BC, Seidl R, Cairns N, Lubec G (1999) Heat-shock protein 70 levels in brain of patients with Down syndrome and Alzheimer’s disease. J Neural Transm Suppl 57:315–322
Morrison-Bogorad M, Zimmerman AL, Pardue S (1995) Heat-shock 70 messenger RNA levels in human brain: correlation with agonal fever. J Neurochem 64:235–246
Kakimura J, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, Taniguchi T, Nomura Y, Gebicke-Haerter PJ, Smith MA, Perry G, Shimohama S (2002) Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J 16:601–603
Calabrese V, Testa G, Ravagna A, Bates TE, Stella AM (2000) HSP70 induction in the brain following ethanol administration in the rat: regulation by glutathione redox state. Biochem Biophys Res Commun 269:397–400
Calabrese V, Bates TE, Giuffrida Stella AM (2000) NO synthase and NO-dependent signal pathways in brain aging and neurodegenerative disorders: the role of oxidant/antioxidant balance. Neurochem Res 65:1315–1341
Yamawaki H, Haendeler J, Berk BC (2003) Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res 93:1029–1033
Cho CG, Kim HJ, Chung SW, Jung KJ, Shim KH, Yu BP, Yodoi J, Chung HY (2003) Modulation of glutathione and thioredoxin systems by calorie restriction during the aging process. Exp Gerontol 38:539–548
Arner ES, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267:6102–6109
Sun QA, Kirnarsky L, Sherman S, Gladyshev VN (2001) Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc Natl Acad Sci USA 98:3673–3678
Bloomfield KL, Osborne SA, Kennedy DD, Clarke FM, Tonissen KF (2003) Thioredoxin-mediated redox control of the transcription factor Sp1 and regulation of the thioredoxin gene promoter. Gene 319:107–116
Kim YC, Yamaguchi Y, Kondo N, Masutani H, Yodoi J (2003) Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene 22:1860–1865
Tanito M, Masutani H, Kim YC, Nishikawa M, Ohira A, Yodoi J (2005) Sulforaphane induces thioredoxin through the antioxidant-responsive element and attenuates retinal light damage in mice. Invest Ophthalmol Vis Sci Mar 46:979–987
Eftekharpour E, Holmgren A, Juurlink BH (2000) Thioredoxin reductase and glutathione synthesis is upregulated by t-butylhydroquinone in cortical astrocytes but not in cortical neurons. Glia 31:241–248
Hintze KJ, Wald KA, Zeng H, Jeffery EH, Finley JW (2003) Thioredoxin reductase in human hepatoma cells is transcriptionally regulated by sulforaphane and other electrophiles via an antioxidant response element. J Nutr 133:2721–2727
Dinkova-Kostova AT, Cheah J, Samouilov A, Zweier JL, Bozak RE, Hicks RJ, Talalay P (2007) Phenolic Michael reaction acceptors: combined direct and indirect antioxidant defenses against electrophiles and oxidants. Med Chem 3:261–268
Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M, Hara S (2005) Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2. J Cell Physiol 203:529–537
Hirota K, Nakamura H, Masutani H, Yodoi J (2002) Thioredoxin superfamily and thioredoxin-inducing agents. Ann NY Acad Sci 957:189–199
Baker AF, Dragovich T, Tate WR, Ramanathan RK, Roe D, Hsu CH, Kirkpatrick DL, Powis G (2006) The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med 147:83–90
Nakamura H, Bai J, Nishinaka Y, Ueda S, Sasada T, Ohshio G, Imamura M, Takabayashi A, Yamaoka Y, Yodoi J (2000) Expression of thioredoxin and glutaredoxin, redox-regulating proteins, in pancreatic cancer. Cancer Detect Prev 24:53–60
Nakamura H, Masutani H, Yodoi J (2002) Redox imbalance and its control in HIV infection. Antioxid Redox Signal 4:455–464
Haapasalo H, Kylaniemi M, Paunul N, Kinnula VL, Soini Y (2003) Expression of antioxidant enzymes in astrocytic brain tumors. Brain Pathol 13:155–164
Berggren MM, Powis G (2001) Alternative splicing is associated with decreased expression of the redox proto-oncogene thioredoxin-1 in human cancers. Arch Biochem Biophys 389:144–149
Biaglow JE, Miller RA (2005) The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther 4:6–13
Bai J, Nakamura H, Kwon YW, Hattori I, Yamaguchi Y, Kim YC, Kondo N, Oka S, Ueda S, Masutani H, Yodoi J (2003) Critical roles of thioredoxin in nerve growth factor-mediated signal transduction and neurite outgrowth in PC12 cells. J Neurosci 23:503–509
Masutani H, Bai J, Kim YC, Yodoi J (2004) Thioredoxin as a neurotrophic cofactor and an important regulator of neuroprotection. Mol Neurobiol 29:229–242
Trigona WL, Mullarky IK, Cao Y, Sordillo LM (2006) Thioredoxin reductase regulates the induction of heme oxygenase-1 expression in aortic endothelial cells. Biochem J 394:207–216
Satoh T, Ishige K, Sagara Y (2004) Protective effects on neuronal cells of mouse afforded by ebselen against oxidative stress at multiple steps. Neurosci Lett 371:1–5
Das KC, Das CK (2000) Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: redox independent functions. Biochem Biophys Res Commun 277:443–447
Ju TC, Chen SD, Liu CC, Yang DI (2005) Protective effects of S-nitrosoglutathione against amyloid beta-peptide neurotoxicity. Free Radic Biol Med 38:938–949
Rauhala P, Andoh T, Yeh K, Chiueh CC (2002) Contradictory effects of sodium nitroprusside and S-nitroso-N-acetylpenicillamine on oxidative stress in brain dopamine neurons in vivo. Ann NY Acad Sci 962:60–72
Lee SY, Andoh T, Murphy DL, Chiueh CC (2003) 17beta-estradiol activates ICI 182, 780-sensitive estrogen receptors and cyclic GMP-dependent thioredoxin expression for neuroprotection. FASEB J 17:947–948
Westphal CH, Dipp MA, Guarente L (2007) A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci 32:555–560
Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K (2008) Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci 65:1049–1058
Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J (2007) Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med 39:335–345
Martin B, Mattson MP, Maudsley S (2006) Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev 5:332–353
Mattson MP (2008) Hormesis defined. Ageing Res Rev 7:1–7
Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L (2007) Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell 6:429–438
Guarente L, Picard F (2005) Calorie restriction—SIR2 connection. Cell 120:473–482
Rodgers JT, Lerin C, Haas W, Cygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1and SIRT1. Nature 434:113–118
Wang F, Nguyen M, Qin FX, Tong Q (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6:505–514
Yang T, Sauve AA (2006) NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J 8:632–643
Smith J (2002) Human Sir2 and the ‘silencing’ of p53 activity. Trends Cell Biol 12:404–406
Hipkiss AR (2008) Energy metabolism, altered proteins, sirtuins and ageing: converging mechanisms? Biogerontology 9:49–55
Hipkiss AR (2007) Could carnosine or related structures suppress Alzheimer’s disease? J Alzheimer’s Dis 11:229–240
Hipkiss AR (2007) On why decreasing protein synthesis can increase lifespan. Mech Ageing Dev 128:412–414
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506
Pallàs M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A (2008) Modulation of sirtuins: new targets for antiageing. Recent Patents CNS Drug Discov 3:61–69
Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450:712–716
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122
Suzuki K, Koike T (2007) Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem Biophys Res Commun 359:665–671
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317:461–462
Qin W, Chachich M, Lane M, Roth G, Bryant M, de Cabo R, Ottinger MA, Mattison J, Ingram D, Gandy S, Pasinetti GM (2006) Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). J Alzheimers Dis 10:417–422
Mayer MP, Bukau B (1999) Molecular chaperones: the busy life of Hsp90. Curr Biol 9:322–325
Deocaris CC, Kaul SC, Wadhwa R (2006) On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 11:116–128
Qiu XB, Shao YM, Miao S, Wang L (2006) The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci 63:2560–2570
James M, Crabbe C, Hepburne-Scott HW (2001) Small heat shock proteins (sHSPs) as potential drug targets. Curr Pharm Biotechnol 2:77–111
Rahman I, Biswas SK, Kirkham PA (2006) Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol 72:1439–1452
Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: the story so far. Eur J Cancer 41:1955–1968
Ravindranath V, Chandrasekhara N (1981–1982) Metabolism of curcumin—studies with [3H]curcumin. Toxicology 22:337–344
Ravindranath V, Chandrasekhara N (1980) Absorption and tissue distribution of curcumin in rats. Toxicology 16:259–265
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK (2007) Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm 330:155–163
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64:353–356
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–2900
Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ (2007) Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem 102:1095–1104
Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP (2007) Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull 30:74–78
Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ (2002) Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 11:105–111
Lin JK, Pan MH, Lin-Shiau SY (2000) Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 13:153–158
Hayeshi R, Mutingwende I, Mavengere W, Masiyanise V, Mukanganyama S (2007) The inhibition of human glutathione S-transferases activity by plant polyphenolic compounds ellagic acid and curcumin. Food Chem Toxicol 45:286–295
Thapliyal R, Maru GB (2001) Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food Chem Toxicol 39:541–547
Basu NK, Ciotti M, Hwang MS, Kole L, Mitra PS, Cho JW, Owens IS (2004) Differential and special properties of the major human UGT1-encoded gastrointestinal UDP-glucuronosyltransferases enhance potential to control chemical uptake. J Biol Chem 279:1429–1441
Oetari S, Sudibyo M, Commandeur JN, Samhoedi R, Vermeulen NP (1996) Effects of curcumin on cytochrome P450 and glutathione S-transferase activities in rat liver. Biochem Pharmacol 51:39–45
Deodhar SD, Sethi R, Srimal RC (1980) Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res 71:632–634
Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10:6847–6854
Sugiyama Y, Kawakishi S, Osawa T (1996) Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol 52:519–525
Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S (1995) Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem 59:1609–1612
Chen WF, Deng SL, Zhou B, Yang L, Liu ZL (2006) Curcumin and its analogues as potent inhibitors of low density lipoprotein oxidation: H-atom abstraction from the phenolic groups and possible involvement of the 4-hydroxy-3-methoxyphenyl groups. Free Radic Biol Med 40:526–535
Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG, Mohan H (2003) Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med 35:475–484
Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P (2007) Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta 1770:989–996
Venkataranganna MV, Rafiq M, Gopumadhavan S, Peer G, Babu UV, Mitra SK (2007) NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene- induced colitis through down-regulation of NFkappa-B and iNOS. World J Gastroenterol 13:1103–1107
Shishodia S, Potdar P, Gairola CG, Aggarwal BB (2003) Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 24:1269–1279
Pan MH, Lin-Shiau SY, Lin JK (2000) Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol 60:1665–1676
Bhattacharyya S, Mandal D, Sen GS, Pal S, Banerjee S, Lahiry L, Finke JH, Tannenbaum CS, Das T, Sa G (2007) Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res 67:362–370
Choi H, Chun YS, Kim SW, Kim MS, Park JW (2006) Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol 70:1664–1671
Abuarqoub H, Green CJ, Foresti R, Motterlini R (2007) Curcumin reduces cold storage-induced damage in human cardiac myoblasts. Exp Mol Med 39:139–148
Jeong GS, Oh GS, Pae HO, Jeong SO, Kim YC, Shin MK, Seo BY, Han SY, Lee HS, Jeong JG, Koh JS, Chung HT (2006) Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp Mol Med 38:393–400
McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ (2006) Curcumin induces heme oxygenase-1 in hepatocytes and is protective in simulated cold preservation and warm reperfusion injury. Transplantation 81:623–626
Rushworth SA, Ogborne RM, Charalambos CA, O’Connell MA (2006) Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem Biophys Res Commun 341:1007–1016
Balogun E, Foresti R, Green CJ, Motterlini R (2003) Changes in temperature modulate heme oxygenase-1 induction by curcumin in renal epithelial cells. Biochem Biophys Res Commun 308:950–955
McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ (2007) Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med 19:165–172
Andreadi CK, Howells LM, Atherfold PA, Manson MM (2006) Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol 69:1033–1040
Rashmi R, Santhosh Kumar TR, Karunagaran D (2003) Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett 538:19–24
Sood A, Mathew R, Trachtman H (2001) Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to shiga toxin. Biochem Biophys Res Commun 283:36–41
Chen YC, Tsai SH, Shen SC, Lin JK, Lee WR (2001) Alternative activation of extracellular signal-regulated protein kinases in curcumin and arsenite-induced HSP70 gene expression in human colorectal carcinoma cells. Eur J Cell Biol 80:213–221
Chen YC, Kuo TC, Lin-Shiau SY, Lin JK (1996) Induction of HSP70 gene expression by modulation of Ca(+2) ion and cellular p53 protein by curcumin in colorectal carcinoma cells. Mol Carcinog 17:224–234
Kato K, Ito H, Kamei K, Iwamoto I (1998) Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones 3:152–160
Fang J, Lu J, Holmgren A (2005) Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem 280:25284–25290
Moi P, Chan K, Asunis I, Cao A, Kan YW (1994) Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA 91:9926–9930
Talalay P (2000) Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors 12:5–11
Nguyen T, Scherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD (2003) Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J 374:337–348
Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM (1995) Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE). Mol Med 1:827–837
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86
Motohashi H, Yamamoto M (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10:549–557
Katsuoka F, Motohashi H, Ishii T, Aburatani H, Engel JD, Yamamoto M (2005) Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol Cell Biol 25:8044–8051
Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA (2005) Nrf2, a multi-organ protector? FASEB J 19:1061–1066
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116
McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD (2006) Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem 281:24756–24768
Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA 99:11908–11913
Dinkova-Kostova AT, Holtzclaw WD, Kensler TW (2005) The role of Keap1 in cellular protective responses. Chem Res Toxicol 18:1779–1791
Kobayashi M, Yamamoto M (2006) Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul 46:113–140
Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S (2004) Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114:1248–1259
Rangasamy T, Guo G, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S (2005) Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202:47–59
Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S (2006) Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 116:984–995
Zhang X, Lu L, Dixon C, Wilmer W, Song H, Chen X, Rovin BH (2004) Stress protein activation by the cyclopentenone prostaglandin 15-deoxy-delta12, 14-prostaglandin J2 in human mesangial cells. Kidney Int 65:798–810
Rokutan K, Miyoshi M, Teshima S, Kawai T, Kawahara T, Kishi K (2000) Phenylarsine oxide inhibits heat shock protein 70 induction in cultured guinea pig gastric mucosal cells. Am J Physiol Cell Physiol 279:1506–1515
Liu H, Lightfoot R, Stevens JL (1996) Activation of heat shock factor by alkylating agents is triggered by glutathione depletion and oxidation of protein thiols. J Biol Chem 271:4805–4812
Tabner BJ, Turnbull S, El-Agnaf O, Allsop D (2001) Production of reactive oxygen species from aggregating proteins implicated in Alzheimer’s disease, Parkinson’s disease and other neurodegenerative diseases. Curr Top Med Chem 1:507–517
Barnham KJ, Cappai R, Beyreuther K, Masters CL, Hill AF (2006) Delineating common molecular mechanisms in Alzheimer’s and prion diseases. Trends Biochem Sci 31:465–472
Hinault M, Ben-Zvi A, Goloubinoff P (2006) Chaperones and proteases: cellular fold-controlling factors of proteins in neurodegenerative diseases and aging. J Mol Neurosci 30:249–265
Butterfield DA (2002) Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res 36:1307–1313
Zhang K, Kaufman RJ (2006) The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66:102–109
Nakamura T, Lipton SA (2007) Molecular mechanisms of nitrosative stress-mediated protein misfolding in neurodegenerative diseases. Cell Mol Life Sci 64:1609–1620
Schroder M (2006) The unfolded protein response. Mol Biotechnol 34:279–290
Schroder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789
Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, Butterfield DA (2006) Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxid Redox Signal 8:1975–1986
Katzman R, Saitoh T (1991) Advances in Alzheimer’s disease. FASEB J 5:278–286
Guix FX, Uribesalgo I, Coma M, Muñoz FJ (2005) The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 76:126–152
Kim DS, Park SY, Kim JK (2001) Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1–42) insult. Neurosci Lett 303:57–61
Ono K, Hasegawa K, Naiki H, Yamada M (2004) Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res 75:742–750
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901
Voelker R (2006) Parkinson disease guidelines aid diagnosis, management. JAMA 295:2126–2128
Przedborski S, Ischiropoulos H (2005) Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antioxid Redox Signal 7:685–693
Hald A, Lotharius J (2005) Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol 193:279–290
Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Proc Natl Acad Sci USA 80:4546–4550
Chiueh CC, Andoh T, Lai AR, Lai E, Krishna G (2000) Neuroprotective strategies in Parkinson’s disease: protection against progressive nigral damage induced by free radicals. Neurotox Res 2:293–310
Rajeswari A (2006) Curcumin protects mouse brain from oxidative stress caused by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Eur Rev Med Pharmacol Sci 10:157–161
Chen J, Tang XP, Zhi JL, Cui Y, Yu HM, Tang EH, Sun SN, Feng JQ, Chen PX (2006) Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis 11:943–953
Mythri RB, Jagatha B, Pradhan N, Andersen J, Bharath MM (2007) Mitochondrial complex I inhibition in Parkinson’s disease: how can curcumin protect mitochondria? Antioxid Redox Signal 9:399–408
Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, Brice A, Koenig M (1996) Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med 335:1169–1175
Campuzano V, Montermini L, Moltò MD, Pianese L, Cossée M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Cañizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–1427
Campuzano V, Montermini L, Lutz Y, Cova L, Hindelang C, Jiralerspong S, Trottier Y, Kish SJ, Faucheux B, Trouillas P, Authier FJ, Dürr A, Mandel JL, Vescovi A, Pandolfo M, Koenig M (1997) Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet 6:1771–1780
Bidichandani SI, Ashizawa T, Patel PI (1998) The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet 62:111–1121
Lamarche JB, Lemieux B, Lieu HB (1984) The neuropathology of “typical” Friedreich’s ataxia in Quebec. Can J Neurol Sci 11:592–600
Delatycki MB, Paris DB, Gardner RJ, Nicholson GA, Nassif N, Storey E, MacMillan JC, Collins V, Williamson R, Forrest SM (1999) Clinical and genetic study of Friedreich ataxia in an Australian population. Am J Med Genet 87:168–174
Montermini L, Richter A, Morgan K, Justice CM, Julien D, Castellotti B, Mercier J, Poirier J, Capozzoli F, Bouchard JP, Lemieux B, Mathieu J, Vanasse M, Seni MH, Graham G, Andermann F, Andermann E, Melançon SB, Keats BJ, Di Donato S, Pandolfo M (1997) Phenotypic variability in Friedreich ataxia: role of the associated GAA triplet repeat expansion. Ann Neurol 41:675–678
Filla A, De Michele G, Cavalcanti F, Pianese L, Monticelli A, Campanella G, Cocozza S (1996) The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet 59:554–560
Sakamoto N, Ohshima K, Montermini L, Pandolfo M, Wells RD (2001) Sticky DNA, a selfassociated complex formed at long GAA*TTC repeats in intron 1 of the frataxin gene, inhibits transcription. J Biol Chem 276:27171–27177
Krasilnikova MM, Mirkin SM (2004) Replication stalling at Friedreich’s ataxia (GAA)n repeats in vivo. Mol Cell Biol 24:2286–2295
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129:823–837
Herman D, Jenssen K, Burnett R, Soragni E, Perlman SL, Gottesfeld JM (2006) Histonedeacetylase inhibitors reverse gene silencing in Friedreich’s ataxia. Nat Chem Biol 2:551–558
Greene E, Mahishi L, Entezam A, Kumari D, Usdin K (2007) Repeat-induced epigenetic changes in intron 1 of the frataxin gene and its consequences in Friedreich ataxia. Nucleic Acids Res 35:3383–3390
Al-Mahdawi S, Pinto RM, Ismail O, Varshney D, Lymperi S, Sandi C, Trabzuni D, Pook M (2008) The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum Mol Genet 17:735–746
Sarsero JP, Li L, Wardan H, Sitte K, Williamson R, Ioannou PA (2003) Upregulation of expression from the FRDA genomic locus for the therapy of Friedreich ataxia. J Gene Med 5:72–81
Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH (2000) Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum Mol Genet 9:275–282
Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M (1997) Kaplan regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276:1709–1712
Foury F, Cazzalini O (1997) Deletion of the yeast homologue of the human gene associated with Friedreich’s ataxia elicits iron accumulation in mitochondria. FEBS Lett 411:373–377
Koutnikova H, Campuzano V, Foury F, Dollé P, Cazzalini O, Koenig M (1997) Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat Genet 16:345–351
Rötig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P (1997) Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 17:215–217
Foury F (1999) Low iron concentration and aconitase deficiency in a yeast frataxin homologue deficient strain. FEBS Lett 456:281–284
Bulteau AL, O’Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI (2004) Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science 305:242–245
Piemonte F, Pastore A, Tozzi G, Tagliacozzi D, Santorelli FM, Carrozzo R, Casali C, Damiano M, Federici G, Bertini E (2001) Glutathione in blood of patients with Friedreich’s ataxia. Eur J Clin Invest 31:1007–1011
Schulz JB, Dehmer T, Schöls L, Mende H, Hardt C, Vorgerd M, Bürk K, Matson W, Dichgans J, Beal MF, Bogdanov MB (2000) Oxidative stress in patients with Friedreich ataxia. Neurology 55:1719–1721
Emond M, Lepage G, Vanasse M, Pandolfo M (2000) Increased levels of plasma malondialdehyde in Friedreich ataxia. Neurology 55:1752–1753
Bradley JL, Homayoun S, Hart PE, Schapira AH, Cooper JM (2004) Role of oxidative damage in Friedreich’s ataxia. Neurochem Res 29:561–567
Santos MM, Ohshima K, Pandolfo M (2001) Frataxin deficiency enhances apoptosis in cells differentiating into neuroectoderm. Hum Mol Genet 10:1935–1944
Sturm B, Stupphann D, Kaun C, Boesch S, Schranzhofer M, Wojta J, Goldenberg H, Scheiber-Mojdehkar B (2005) Recombinant human erythropoietin: effects on frataxin expression in vitro. Eur J Clin Invest 35:711–717
Boesch S, Sturm B, Hering S, Goldenberg H, Poewe W, Scheiber-Mojdehkar B (2007) Friedreich’s ataxia: clinical pilot trial with recombinant human erythropoietin. Ann Neurol 62:521–524
Cooper JM, Schapira AH (2003) Friedreich’s ataxia: disease mechanisms, antioxidant and coenzyme Q10 therapy. Biofactors 18:163–171
Cooper JM, Schapira AH (2007) Friedreich’s ataxia: coenzyme Q10 and vitamin E therapy. Mitochondrion 7:127–135
Lodi R, Hart PE, Rajagopalan B, Taylor DJ, Crilley JG, Bradley JL, Blamire AM, Manners D, Styles P, Schapira AH, Cooper JM (2001) Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich’s ataxia. Ann Neurol 49:590–596
Hart PE, Lodi R, Rajagopalan B, Bradley JL, Crilley JG, Turner C, Blamire AM, Manners D, Styles P, Schapira AH, Cooper JM (2005) Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol 62:621–626
Rustin P, von Kleist-Retzow JC, Chantrel-Groussard K, Sidi D, Munnich A, Rötig A (1999) Effect of idebenone on cardiomyopathy in Friedreich’s ataxia: a preliminary study. Lancet 354:477–479
Hausse AO, Aggoun Y, Bonnet D, Sidi D, Munnich A, Rötig A, Rustin P (2002) Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia. Heart 87:346–349
Buyse G, Mertens L, Di Salvo G, Matthijs I, Weidemann F, Eyskens B, Goossens W, Goemans N, Sutherland GR, Van Hove JL (2003) Idebenone treatment in Friedreich’s ataxia: neurological, cardiac, and biochemical monitoring. Neurology 60:1679–1681
Mariotti C, Solari A, Torta D, Marano L, Fiorentini C, Di Donato S (2003) Idebenone treatment in Friedreich patients: one-year-long randomized placebo-controlled trial. Neurology 60:1676–1679
Di Prospero NA, Baker A, Jeffries N, Fischbeck KH (2007) Neurological effects of high-dose idebenone in patients with Friedreich’s ataxia: a randomised, placebo-controlled trial. Lancet Neurol 6:878–886
Jauslin ML, Meier T, Smith RA, Murphy MP (2003) Mitochondria-targeted antioxidants protect Friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17:1972–1974
Calabrese V, Scapagnini G, Ravagna A, Giuffrida Stella AM, Butterfield DA (2002) Molecular chaperones and their roles in neural cell differentiation. Dev Neurosci 24:1–13
Al-Omar FA, Nagi MN, Abdulgadir MM, Al Joni KS, Al-Majed AA (2006) Immediate and delayed treatments with curcumin prevents forebrain ischemia-induced neuronal damage and oxidative insult in the rat hippocampus. Neurochem Res 31:611–618
Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY (2005) Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res 82:138–148
Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES (2002) Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res 46:273–279
Evans AM, Fornasini G (2003) Pharmacokinetics of L-carnitine. Clin Pharmacokinet 42:941–967
Rebouche CJ (2004) Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann NY Acad Sci 1033:30–41
Lombard KA, Olson AL, Nelson SE, Rebouche CJ (1989) Carnitine status of lactoovovegetarians and strict vegetarian adults and children. Am J Clin Nutr 50:301–306
Brass EP, Hoppel CL, Hiatt WR (1994) Effect of intravenous L-carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clin Pharmacol Ther 55:681–692
Gross CJ, Savaiano DA (1993) Effect of development and nutritional state on the uptake, metabolism and release of free and acetyl-L-carnitine by the rodent small intestine. Biochim Biophys Acta 1170:265–274
Parnetti L, Gaiti A, Mecocci P, Cadini D, Senin U (1992) Pharmacokinetics of IV and oral acetyl-L-carnitine in a multiple dose regimen in patients with senile dementia of Alzheimer type. Eur J Clin Pharmacol 42:89–93
Kelly JG, Hunt S, Doyle GD, Laher MS, Carmody M, Marzo A, Arrigoni Martelli E (1990) Pharmacokinetics of oral acetyl-L-carnitine in renal impairment. Eur J Clin Pharmacol 38:309–312
McDaniel MA, Maier SF, Einstein GO (2003) “Brain-specific” nutrients: a memory cure? Nutrition 19:957–975
Abdul HM, Calabrese V, Calvani M, Butterfield DA (2006) Acetyl-L-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1–42-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. J Neurosci Res 84:398–408
Calabrese V, Colombrita C, Sultana R, Scapagnini G, Calvani M, Butterfield DA, Stella AM (2006) Redox modulation of heat shock protein expression by acetylcarnitine in aging brain: relationship to antioxidant status and mitochondrial function. Antioxid Redox Signal 8:404–416
Rai G, Wright G, Scott L, Beston B, Rest J, Exton-Smith AN (1990) Double-blind, placebo controlled study of acetyl-l-carnitine in patients with Alzheimer’s dementia. Curr Med Res Opin 11:638–647
Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, Tettamanti M, Frattura L, Tiraboschi P, Comelli M (1991) Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology 41:1726–1732
Thal LJ, Carta A, Clarke WR, Ferris SH, Friedland RP, Petersen RC, Pettegrew JW, Pfeiffer E, Raskind MA, Sano M, Tuszynski MH, Woolson RF (1996) A 1-year multicenter placebo-controlled study of acetyl-L-carnitine in patients with Alzheimer’s disease. Neurology 47:705–711
Thal LJ, Calvani M, Amato A, Carta A (2000) A 1-year controlled trial of acetyl-l-carnitine in early-onset AD. Neurology 55:805–810
Calabrese V, Ravagna A, Colombrita C, Guagliano E, Scapagnini G, Calvani M, Butterfield DA, Giuffrida Stella AM (2005) Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res 79:509–521
Snyder SH (1980) Brain peptides as neurotransmitters. Science 209:976–983
Abe H (2000) Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc) 65:757–765
Hipkiss AR, Preston JE, Himsworth DT, Worthington VC, Keown M, Michaelis J, Lawrence J, Mateen A, Allende L, Eagles PA, Abbott NJ (1998) Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann NY Acad Sci 854:37–53
Bakardjiev A (1997) Biosynthesis of carnosine in primary cultures of rat olfactory bulb. Neurosci Lett 227:115–118
Teufel M, Saudek V, Ledig JP, Bernhardt A, Boularand S, Carreau A, Cairns NJ, Carter C, Cowley DJ, Duverger D, Ganzhorn AJ, Guenet C, Heintzelmann B, Laucher V, Sauvage C, Smirnova T (2003) Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem 278:6521–6531
Bauer K (2004) X-His dipeptidase. In: Barret AJ, Rawlings ND, Woessner JF (eds) Handbook of proteolytic enzymes, 2nd edn. Elsevier, Amsterdam, pp 1023–1024
Bauer K (2004) Cytosol non-specific dipeptidase. In: Barret AJ, Rawlings ND, Woessner JF (eds) Handbook of Proteolytic Enzymes, 2nd edn. Amsterdam, Elsevier, pp 1020–1022
De Marchis S, Modena C, Peretto P, Giffard C, Fasolo A (2000) Carnosine-like immunoreactivity in the central nervous system of rats during postnatal development. J Comp Neurol 426:378–390
Bauer K (2005) Carnosine and homocarnosine, the forgotten, enigmatic peptides of the brain. Neurochem Res 30:1339–1345
McFarland GA, Holliday R (1994) Retardation of senescence in cultured human diploid fibroblasts by carnosine. Exp Cell Res 212:167–175
Yuneva AO, Kramarenko GG, Vetreshchak TV, Gallant S, Boldyrev AA (2002) Effect of carnosine on Drosophila melanogaster lifespan. Bull Exp Biol Med 133:559–561
Yuneva AO, Bulygina ER, Gallant S, Kramarenko GG, Stvolinsky SL, Semyonova ML, Boldyrev AA (1999) Effect of carnosine on age-induced changes in senescence-accelerated mice. J Anti-Aging Med 2:337–342
Babizhayev MA (2006) Biological activities of the natural imidazole-containing peptidomimetics n-acetylcarnosine, carcinine and l-carnosine in ophthalmic and skin care products. Life Sci 78:2343–2357
Lee Y, Hsu C, Lin M, Liu K, Yin M (2005) Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol 512:145–150
Rashid I, van Reyk DM, Davies MJ (2007) Carnosine and its constituents inhibit glycation of low-density lipoproteins that promotes foam cell formation in vitro. FEBS Letts 581:1067–1070
Sauerhofer S, Yuan G, Braun GS, Deizer M, Neumaier M, Gretz N, Floege J, Kriz W, van der Woude F, Moeller MJ (2007) L-Carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 56:2425–2432
Hipkiss AR (2005) Glycation, aging and carnosine: are carnivorous diets beneficial? Mech Ageing Dev 126:1034–1039
Huang Y, Duan J, Chen H, Chen M, Chen G (2005) Separation and determination of carnosine-related peptides using capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 26:593–599
Hipkiss AR (2005) Could carnosine suppress zinc-mediated proteasome inhibition and neurodegeneration? Therapeutic potential of a non-toxic but non-patentable dipeptide. Biogerontology 6:147–149
Hipkiss AR (2007) Could carnosine or related structures suppress Alzheimer’s disease? J Alzheimer’s Dis 11:229–240
Dobrota D, Fedorova T, Stvolinsky S, Babusikova E, Likavcanova K, Drgova A, Strapkova A, Boldyrev A (2005) Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: after-stroke-effect. Neurochem Res 30:1283–1288
Stvolinsky S, Kukley M, Dobrota D, Mezesova V, Boldyrev A (2000) Carnosine protects rats under global ischemia. Brain Res Bull 53:445–448
Tang S, Arumugam TV, Cutler RG, Jo D, Magnus T, Chan SL, Mughal MR, Telljohann RS, Nassar M, Ouyang X, Calderan A, Ruzza P, Guiotto A, Mattson MP (2007) J Neurochem 101:729–736
Pubill D, Verdaguer E, Sureda FX, Camins A, Pallas M, Camarasa J, Escubedo E (2002) Carnosine prevents methamphetamine-induced gliosis but not dopamine terminal loss in rats. Eur J Pharmacol 448:165–168
Dukic-Stefanovic S, Schinzel R, Riederer P, Munch G (2001) AGES in brain ageing: AGE-inhibitors as neuroprotective and anti-dementia drugs? Biogerontology 2:19–34
Trombley PQ, Horning MS, Blakemore LJ (1998) Carnosine modulates zinc and copper effects on amino acid receptors and synaptic transmission. Neuroreport 9:3503–3507
Horning MS, Blakemore LJ, Trombley PQ (2000) Endogenous mechanisms of neuroprotection: role of zinc, copper, and carnosine. Brain Res 852:56–61
Preston JE, Hipkiss AR, Himsworth DT, Romero IA, Abbott JN (1998) Toxic effects of beta-amyloid(25–35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and beta-alanine. Neurosci Lett 242:105–108
Fu Q, Dai H, Hu W, Fan Y, Zhang Y, Chen Z (2008) Carnosine protects against Aβ-42-induced neurotoxicity in differentiated rat PC12 cells. Cell Mol Neurobiol 28:307–316
Shen Y, Hu W, Dai H, Fu Q, Wei E, Luo J, Chen Z (2007) Carnosine protects against NMDA-induced neurotoxicity in differentiated rat PC12 cells through carnosine-histidine-histamine pathway and H1/H3 receptors. Biochem Pharmacol 73:709–717
Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG (2007) Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 32:213–224
Fontana M, Pinnen F, Lucente G, Pecci L (2002) Prevention of peroxynitrite-dependent damage by carnosine and related sulphonamido pseudodipeptides. Cell Mol Life Sci 59:546–551
Severina IS, Bussygina OG, Pyatakova NV (2000) Carnosine as a regulator of soluble guanylate cyclase. Biochemistry (Mosc) 65:783–788
Calabrese V, Colombrita C, Guagliano E, Sapienza M, Ravagna A, Cardile V, Scapagnini G, Santoro AM, Mangiameli A, Butterfield DA, Giuffrida Stella AM, Rizzarelli E (2005) Protective effect of carnosine during nitrosative stress in astroglial cell cultures. Neurochem Res 30:797–807
Nicoletti VG, Santoro AM, Grasso G, Vagliasindi LI, Giuffrida ML, Cuppari C, Spina Purrello V, Stella Giuffrida AM, Rizzarelli E (2007) Carnosine interaction with nitric oxide and astroglial protection. J Neurosci Res 85:2239–2245
La Mendola D, Sortino S, Vecchio G, Rizzarelli E (2002) Synthesis of new carnosine derivatives of β-cyclodextrin and their hydroxyl scavenger ability. Helv Chim Acta 85:1633–1643
Bonomo RP, Bruno V, Conte E, De Guidi G, La Mendola D, Maccarrone G, Nicoletti F, Rizzarelli E, Sortino S, Vecchio G (2003) Potentiometric, spectroscopic and antioxidant activity studies of SOD mimics containing carnosine. J Chem Soc Dalton Trans 4406–4415
Mineo P, Vitalini D, La Mendola D, Rizzarelli E, Scamporrino E, Vecchio G (2004) ESI-MS and spectroscopic investigations on 6A, 6D-di-(β-alanyl-L-histidine)-6A, 6D-dideoxy-β-cyclodextrin and 6A, 6C-di-(β-alanyl-L-histidine)-6A, 6C-dideoxy-β-cyclodextrin and their copper(II) complexes. J Inorg Biochem 98:254–265
Bellia F, La Mendola D, Maccarrone G, Mineo P, Vitalizi D, Scamporrino E, Sortino S, Vecchio G, Rizzarelli E (2007) Copper(II) complexes with β-cyclodextrin-homocarnosine conjugates and their antioxidant activity. Inorg Chim Acta 360:949–954
Amorini AM, Bellia F, Di Pietro V, Giardina B, La Mendola D, Lazzarino G, Sortino S, Tavazzi B, Rizzarelli E, Vecchio G (2007) Synthesis and antioxidant activity of new homocarnosine β-cyclodextrin conjugates. Eur J Med Chem 42:910–920
Bellia F, Amorini AM, La Mendola D, Vecchio G, Gavazzi B, Giardina B, Di Pietro V, Lazzarino G, Rizzarelli E (2008) New glycosidic derivatives of histidine-containing dipeptides with antioxidant properties and resistant to carnosinase activity. Eur J Med Chem 43:373–380
Adlard PA, Bush AI (2006) Metals and Alzheimer’s disease. J Alzheimer’s Dis 10:145–163
Chrouch PJ, White AR, Bush AI (2007) The modulation of metal bio-availability as a therapeutic strategy for the treatment of Alzheimer’s disease. FEBS J 274:375–3783
Wenzel E, Somoza V (2005) Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res 49:472–481
Gescher AJ, Steward WP (2003) Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev 12:953–957
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr, Walle UK (2004) High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 32:1377–1382
Soleas GJ, Angelini M, Grass L, Diamandis EP, Goldberg DM (2001) Absorption of trans-resveratrol in rats. Methods Enzymol 335:145–154
Jannin B, Menzel M, Berlot JP, Delmas D, Lançon A, Latruffe N (2004) Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: plasmatic protein binding and cell uptake. Biochem Pharmacol 68:1113–1118
Zhou S, Koh HL, Gao Y, Gong ZY, Lee EJ (2004) Herbal bioactivation: the good, the bad and the ugly. Life Sci 74:935–968
Cao Z, Li Y (2004) Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol 489:39–48
Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM (2002) Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 33:387–398
Kim YA, Kim GY, Park KY, Choi YH (2007) Resveratrol inhibits nitric oxide and prostaglandin E2 production by lipopolysaccharide-activated C6 microglia. J Med Food 10:218–224
Anekonda TS (2006) Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Rev 52:316–326
Jang JH, Surh YJ (2003) Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med 34:1100–1110
Bastianetto S, Brouillette J, Quirion R (2007) Neuroprotective effects of natural products: interaction with intracellular kinases, amyloid peptides and a possible role for transthyretin. Neurochem Res 32:1720–1725
Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem 280:37377–37382
Bastianetto S, Zheng WH, Quirion R (2000) Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol 131:711–720
Sharma M, Gupta YK (2002) Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci 71:2489–2498
Pallàs M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A (2008) Modulation of sirtuins: new targets for antiageing. Recent Patents CNS Drug Discov 3:61–69
Anekonda TS, Reddy PH (2006) Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem 96:305–313
Chen CY, Jang JH, Li MH, Surh YJ (2005) Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun 331:993–1000
Zhuang H, Kim YS, Koehler RC, Doré S (2003) Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann NY Acad Sci 993:276–286
Takahashi M, Doré S, Ferris CD, Tomita T, Sawa A, Wolosker H, Borchelt DR, Iwatsubo T, Kim SH, Thinakaran G, Sisodia SS, Snyder SH (2000) Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron 28:461–473
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Giuffrida Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8:766–775
Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, Bartholomew JC, Ames BN (2002) Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci USA 99:1870–1875
Hagen TM, Moreau R, Suh JH, Visioli F (2002) Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann NY Acad Sci 959:491–507
Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN (2002) Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci USA 99:2356–2361
Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN (2008) Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J 22:703–712
Milgram NW, Araujo JA, Hagen TM, Treadwell BV, Ames BN (2007) Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J 21:3756–3762
Mattson MP (2008) Hormesis defined. Ageing Res Rev 7:1–7
Calabrese E (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 14:1
Calabrese EJ (2008) Another california milestone: the first application of hormesis in litigation and regulation. Int J Toxicol 27:31–33
Acknowledgments
This work was supported by grants of MIUR, FIRB RBNE03PX83, and FIRB RBRN07BMCT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Anna Maria Giuffrida-Stella.
Rights and permissions
About this article
Cite this article
Calabrese, V., Cornelius, C., Mancuso, C. et al. Cellular Stress Response: A Novel Target for Chemoprevention and Nutritional Neuroprotection in Aging, Neurodegenerative Disorders and Longevity. Neurochem Res 33, 2444–2471 (2008). https://doi.org/10.1007/s11064-008-9775-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9775-9