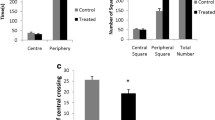
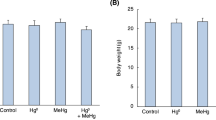
During the early postnatal period the central nervous system (CNS) is extremely sensitive to external agents. The present study aims at the investigation of critical phases where methylmercury (MeHg) induces cerebellar toxicity during the suckling period in mice. Animals were treated with daily subcutaneous injections of MeHg (7 mg/kg of body weight) during four different periods (5 days each) at the early postnatal period: postnatal day (PND) 1–5, PND 6–10, PND 11–15, or PND 16–20. A control group was treated with daily subcutaneous injections of a 150 mM NaCl solution (10 ml/kg of body weight). Subjects exposed to MeHg at different postnatal periods were littermate. At PND 35, behavioral tests were performed to evaluate spontaneous locomotor activity in the open field and motor performance in the rotarod task. Biochemical parameters related to oxidative stress (levels of glutathione and thiobarbituric acid reactive substances, as well as glutathione peroxidase and glutathione reductase activity) were evaluated in cerebellum. Hyperlocomotor activity and high levels of cerebellar thiobarbituric acid reactive substances were observed in animals exposed to MeHg during the PND 11–15 or PND 16–20 periods. Cerebellar glutathione reductase activity decreased in MeHg-exposed animals. Cerebellar glutathione peroxidase activity was also decreased after MeHg exposure and the lowest enzymatic activity was found in animals exposed to MeHg during the later days of the suckling period. In addition, low levels of cerebellar glutathione were found in animals exposed to MeHg during the PND 16–20 period. The present results show that the postnatal exposure to MeHg during the second half of the suckling period causes hyperlocomotor activity in mice and point to this phase as a critical developmental stage where mouse cerebellum is a vulnerable target for the neurotoxic and pro-oxidative effects of MeHg.


Similar content being viewed by others
References
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury–current exposures and clinical manifestations. N Engl J Med 349:1731–1737
Sirois JE, Atchison WD (2000) Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol Appl Pharmacol 167:1–11
Ou YC, White CC, Krejsa CM, Ponce RA, Kavanagh TJ, Faustman EM (1999) The role of intracellular glutathione in methylmercury-induced toxicity in embryonic neuronal cells. Neurotoxicology 20:793–804
Aschner M, Yao CP, Allen JW, Tan KH (2000) Methylmercury alters glutamate transport in astrocytes. Neurochem Int 37:199–206
Farina M, Dahm KC, Schwalm FD, Brusque AM, Frizzo ME, Zeni G, Souza DO, Rocha JB (2003a) Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol Sci 73:135–140
Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JB, Frizzo ME, Souza DO, Farina M (2004) Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci 81:172–178
Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP (2004) Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol 44:87–110
Rasmussen EB, Newland MC (2001) Developmental exposure to methylmercury alters behavioral sensitivity to d-amphetamine and pentobarbital in adult rats. Neurotoxicol Teratol 23:45–55
Newland MC, Reile PA, Langston JL (2004) Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol 26:179–194
Weiss B, Stern S, Cox C, Balys M (2005) Perinatal and lifetime exposure to methylmercury in the mouse: behavioral effects. Neurotoxicology 26:675–690
Stern S, Cox C, Cernichiari E, Balys M, Weiss B (2001) Perinatal and lifetime exposure to methylmercury in the mouse: blood and brain concentrations of mercury to 26 months of age. Neurotoxicology 22:467–477
Gottlieb A, Keydar I, Epstein HT (1977) Rodent brain growth stages: an analytical review Biol Neonate 32:166–176
Aschner M (1996) Methylmercury in astrocytes – what possible significance? Neurotoxicology 17:93–106
Choi BH, Yee S, Robles M (1996) The effects of glutathione glycoside in methyl mercury poisoning. Toxicol Appl Pharmacol 141:357–364
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Khan JY, Black SM (2003) Developmental changes in murine brain antioxidant enzymes. Pediatr Res 54:77–82
Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, Rocha JB, Souza DO (2003b) Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicol Lett 144:351–357
Goulet S, Dore FY, Mirault ME (2003) Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol 25:335–347
Farina M, Franco JL, Ribas CM, Meotti FC, Pizzolatti MG, Dafre AL, Santos ARS (2005) Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol 57:1503–1508
Duhan NW, Miya TS (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc 46:208–209
Klein R, Herman SP, Brubaker PE, Lucier GW, Krigman MR (1972) A model of acute methyl mercury intoxication in rats. Arch Pathol 93:408–418
Sanfeliu C, Sebastia J, Cristofol R, Rodriguez-Farre E (2003) Neurotoxicity of organomercurial compounds. Neurotox Res 5:283–305
Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ (1997) Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 19:417–428
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Farina M, Brandão R, de Lara FS, Pagliosa LB, Soares FA, Souza DO, Rocha JB (2003c) Profile of nonprotein thiols, lipid peroxidation and delta-aminolevulinate dehydratase activity in mouse kidney and liver in response to acute exposure to mercuric chloride and sodium selenite. Toxicology 184:179–187
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Flohe L (1971) Glutathione peroxidase: enzymology and biological aspects. Klin Wochenschr 49:669–683
Gul M, Kutay FZ, Temocin S, Hanninen O (2000) Cellular and clinical implications of glutathione. Indian J Exp Biol 38:625–634
Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB (1997) Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol 52:261–281
Tiffany-Castiglioni E, Guerri C, Aschner M, Matsushima GK, O’Callaghan JP, Streit WJ (2001) Roles of glia in developmental neurotoxicity: session VI summary and research needs. Neurotoxicology 22:567–573
Raps SP, Lai JC, Hertz L, Cooper AJ (1989) Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res 493:398–401
Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ (1994) Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem 62:45–53
Booth RF, Patel TB, Clark JB (1980) The development of enzymes of energy metabolism in the brain of a precocial (guinea pig) and non-precocial (rat) species. J Neurochem 34:17–25
Sakamoto M, Nakano A, Kajiwara Y, Naruse I, Fujisaki T (1993) Effects of methyl mercury in postnatal developing rats. Environ Res 61:43–50
Dietrich MO, Mantese CE, Anjos GD, Souza DO, Farina M (2005) Motor impairment induced by oral exposure to methylmercury in adult mice. Environ Toxicol Pharmacol 19:169–175
Farina M, Cereser V, Portela LV, Mendez A, Porciúncula LO, Fornaguera J, Gonçalves CA, Wofchuk ST, Rocha JBT, Souza DO (2005c) Methylmercury increases S100B content in rat cerebrospinal fluid. Environ Toxicol Pharmacol 19:249–253
Fredriksson A, Dencker L, Archer T, Danielsson BR (1977) Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changes in adult rats. Neurotoxicol Teratol 18:129–134
Dare E, Fetissov S, Hokfelt T, Hall H, Ogren SO, Ceccatelli S (2003) Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Naunyn Schmiedebergs Arch Pharmacol 367:500–508
Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW (2003) Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet 361:1686–1692
Acknowledgements
This study was supported by grants from CNPq to M. Farina (475329/2004–0). J. Strigari was a recipient of a CNPq/PIBIC fellowship.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stringari, J., Meotti, F.C., Souza, D.O. et al. Postnatal Methylmercury Exposure Induces Hyperlocomotor Activity and Cerebellar Oxidative Stress in Mice: Dependence on the Neurodevelopmental Period. Neurochem Res 31, 563–569 (2006). https://doi.org/10.1007/s11064-006-9051-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9051-9