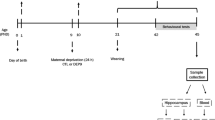
We studied effects of restraint-induced stress and morphine co-administration within the prenatal period and of re-exposure to stress at the end of infancy on the body mass and pentylenetetrazol-induced epileptic manifestations in rats. Pregnant rats were divided into six groups (control, restraint stressed, saline, morphine, stress+saline, and stress+morphine). In the stressed groups, pregnant rats were subjected to restraint stressing twice per day for three consecutive days (starting on pregnancy day 15). Rats in saline and morphine groups received saline and morphine subcutaneously on the same days. In the morphine/saline+stressed groups, rats were exposed to stress and received morphine/saline simultaneously. Control rats were used intact. The pups were weighed at postnatal days (PD) 1, 15, and 22. On P22, half of the pups were re-exposed to stress; then, pentylenetetrazol (PTZ)-induced seizures were recorded. The offspring body mass was significantly smaller in stressed, morphine, and stressed+morphine groups compared to the control. The time to onset of the first tonic-clonic seizure was shorter, while the duration and number of tonic-clonic attacks were significantly greater in the stressed+morphine group compared to other groups. Re-exposure to stress decreased the number of clonic seizures. The number of leg-opening and tail rigidity episodes was smaller in female offspring compared to male ones. Co-administration of restraint stress and morphine within the prenatal period reduces the offspring body mass and increases the seizure vulnerability more severely compared to the respective individual effects In addition, prenatal stress exerts stronger effects on the neural development and epileptic behaviors of the offspring than postnatal stress.
Similar content being viewed by others
References
H. E. Edwards, D. Dortok, J. Tam, et al., “Prenatal stress alters seizure thresholds and the development of kindled seizures in infant and adult rats,” Horm. Behav., 42, 437-447 (2002).
R. Slamberova, B. Schutova, I. Matejovska, et al., “Effects of a single postnatal methamphetamine administration on NMDA-induced seizures are sex- and prenatal exposure-specific,” Naunyn Schmiedebergs Arch. Pharmacol., 380, 109-114 (2009).
M. Weinstock, “The long-term behavioural consequences of prenatal stress,” Neurosci. Biobehav. Rev., 32, 1073-1086 (2008).
H. Koyuncuoglu and F. Aricioglu, “Prenatal exposure to morphine or naloxone intensifies morphine dependence at maturity,” Pharmacol. Biochem. Behav., 44, 939-941 (1993).
C. J. Schindler, J. Veliskova, R. Slamberova, and I. Vathy, “Prenatal morphine exposure alters susceptibility to bicuculline seizures in a sex- and agespecific manner,” Brain Res. Dev. Brain Res., 121, 119-122 (2000).
I. Vathy, J. Veliskova, and S. L. Moshe, “Prenatal morphine exposure induces age-related changes in seizure susceptibility in male rats,” Pharmacol. Biochem. Behav., 60, 635-638 (1998).
L. Velisek, J. Veliskova, S. L. Moshe, and I. Vathy, “Prenatal morphine exposure alters ovarian steroid hormonal regulation of seizure susceptibility,” Brain Res., 796, 247-256 (1998).
I. Vathy, “Prenatal opiate exposure: long-term CNS consequences in the stress system of the offspring,” Psychoneuroendocrinology, 27, 273-283 (2002).
I. Ahmad and B. J. Pleuvry, “Interactions between opioid drugs and Propofol in laboratory models of seizures,” Br. J. Anaesth., 74, 311-314 (1995).
M. Massotti and K. Gale, “Electroencephalographic evidence for a dose-related biphasic effect of morphine on bicuculline-induced seizures in the rat,” Epilepsy Res., 4, 81-89 (1989).
H. Shafaroodi, M. Samini, L. Moezi, et al., “The interaction of cannabinoids and opioids on pentylenetetrazole-induced seizure threshold in mice,” Neuropharmacology, 47, 390-400 (2004).
T. M. Tzschentke, “Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues,” Prog. Neurobiol., 56, 613-672 (1998).
A. Becker, G. Grecksch, W. Thiemann, and V. Hollt, “Pentylenetetrazol-kindling modulates stimulated dopamine release in the nucleus accumbens of rats,” Pharmacol. Biochem. Behav., 66, 425-428 (2000).
M. Gholami and E. Saboory, “Morphine exposure induces age-dependent alterations in pentylenetetrazole-induced epileptic behaviors in prepubertal rats,” Dev. Psychobiol., 55, 881-887 (2013).
P. J. Brunton and J. A. Russell, “Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects,” J. Neuroendocrinol., 22, 258-271 (2010).
H. Caspers and E. J. Speckmann, “Cerebral pO2 , pCO2 and pH: changes during convulsive activity and their significance for spontaneous arrest of seizures,” Epilepsia, 13, 699-725 (1972).
R. Ahmadzadeh, E. Saboory, S. Roshan-Milani, and A. A. Pilehvarian, “Predator and restraint stress during gestation facilitates pilocarpine-induced seizures in prepubertal rats,” Dev. Psychobiol., 53, 806-812 (2011).
L. Ebrahimi, E. Saboory, S. Roshan-Milani, and P. Hashemi, “Effect of prenatal forced-swim stress and morphine co-administration on pentylentetrazol-induced epileptic behaviors in infant and prepubertal rats,” Dev. Psychobiol., 56, 1179-1186 (2014).
P. Hashemi, L. Ebrahimi, E. Saboory, and S. Roshan-Milani, “Effect of restraint stress during gestation on pentylenetetrazol-induced epileptic behaviors in rat offspring,” Iran. J. Basic Med. Sci., 16, 979-984 (2013).
E. Saboory, R. Ahmadzadeh, and S. Roshan-Milani, “Prenatal exposure to restraint or predator stresses attenuates field excitatory postsynaptic potentials in infant rats,” Int. J. Dev. Neurosci., 29, 827-831 (2011).
M. M. Sadaghiani and E. Saboory, “Prenatal stress potentiates pilocarpine-induced epileptic behaviors in infant rats both time and sex dependently,” Epilepsy Behav., 18, 166-170 (2010).
E. Tavassoli, E. Saboory, M. Teshfam, et al., “Effect of prenatal stress on density of NMDA receptors in rat brain,” Int. J. Dev. Neurosci., 31, 790-795 (2013).
M. Gholami and E. Saboory, “Morphine exposure induces age-dependent alterations in pentylenetetrazole-induced epileptic behaviors in prepubertal rats,” Dev. Psychobiol., 55, 881-887 (2013).
M. Gholami, E. Saboory, and H. R. Khalkhali, “Chronic morphine and tramadol re-exposure induced an antianxiety effect in prepubertal rats exposed neonatally to the same drugs,” Clin. Exp. Pharmacol. Physiol., 41, 838-843 (2014).
M. Gholami, E. Saboory, S. Mehraban, et al., “Time dependent antinociceptive effects of morphine and tramadol in the hot plate test: using different methods of drug administration in female rats,” Clin. Exp. Pharmacol. Physiol., 14, 303-311 (2015).
E. Saboory, M. Derchansky, M. Ismaili, et al., “Mechanisms of morphine enhancement of spontaneous seizure activity,” Anesth. Analg., 105, 1729-1735, (2007).
E. Saboory, M. Gholami, S. Zare, and S. Roshan-Milani, “The long-term effects of neonatal morphine administration on the pentylenetetrazol seizure model in rats: the role of hippocampal cholinergic receptors in adulthood,” Dev. Psychobiol., 56, 498-509 (2014).
C. Laborie, I. Dutriez-Casteloot, V. Montel, et al., “Prenatal morphine exposure affects sympathoadrenal axis activity and serotonin metabolism in adult male rats both under basal conditions and after an ether inhalation stress,” Neurosci. Lett., 381, 211-216 (2005).
J. Lesage, M. Grino, F. Bernet et al., “Consequences of prenatal morphine exposure on the hypothalamo-pituitary-adrenal axis in the newborn rat: effect of maternal adrenalectomy,” J. Neuroendocrinol., 10, 331-342 (1998).
A. Rimanoczy, R. Slamberova, M. A. Riley, and I. Vathy, “Adrenocorticotropin stress response but not glucocorticoid-negative feedback is altered by prenatal morphine exposure in adult male rats,” Neuroendocrinology, 78, 312-320 (2003).
J. McDowell and I. Kitchen, “Development of opioid systems: peptides, receptors and pharmacology,” Brain Res., 434, 397-421 (1987).
M. Weinstock, “Alterations induced by gestational stress in brain morphology and behaviour of the offspring,” Prog. Neurobiol., 65, 427-451 (2001).
B. Heshmatian, S. Roshan-Milani, and E. Saboory, “Prenatal acute stress attenuated epileptiform activities in neonate mice,” Yakhteh Med. J., 12, 81-86 (2010).
R. J. Racine, “Modification of seizure activity by electrical stimulation. II. Motor seizure,” EEG Clin. Neurophysiol., 32, 281-294 (1972).
J. J. Hablitz and U. Heinemann, “Extracellular K+ and Ca2+ changes during epileptiform discharges in the immature rat neocortex,” Brain Res., 433, 299-303 (1987).
S. L. Moshe and B. J. Albala, “Maturational changes in postictal refractoriness and seizure susceptibility in developing rats,” Annu. Neurol., 13, 552-557 (1983).
J. Veliskova and L. S. Velisek, “Picrotoxin-induced tonic-clonic seizures and lethality are decreased by MK-801 in developing rats,” Pharmacol. Biochem. Behav., 43, 291-295 (1992).
S. L. Moshe, D. S. Garant, E. F. Sperber, et al., “Ontogeny and topography of seizure regulation by the substantia nigra,” Brain Dev., Suppl. 17, 61-72 (1995).
M. M. Sadaghiani and E. Saboory, “Prenatal stress potentiates pilocarpine-induced epileptic behaviors in infant rats both time and sex dependently,” Epilepsy Behav., 18, 166-170 (2010)
J. Mairesse, J. Lesage, C. Breton, et al., “Maternal stress alters endocrine function of the feto-placental unit in rats,” Am. J. Physiol. Endocrinol. Metab., 292, E1526-33 (2007).
B. A. Gosnell and D. D. Krahn, “The effects of continuous morphine infusion on diet selection and body weight,” Physiol. Behav., 54, 853-859 (1993).
L. Ebrahimi, E. Saboory, S. Roshan-Milani, and P. Hashemi,. “Effect of prenatal forced-swim stress and morphine co-administration on pentylentetrazol-induced epileptic behaviors in infant and prepubertal rats,” Dev. Psychobiol., 56, 1179-1186 (2014).
A. Fodor, J. Timar, and D. Zelena, “Behavioral effects of perinatal opioid exposure,” Life Sci., 104, 1-8 (2014).
M. A. Galic, N. M. Fournier, and L. J. Martin, “Alpha2-adrenergic inhibition prevents the accompanied anticonvulsant effect of swim stress on behavioral convulsions induced by lithium and pilocarpine,” Pharmacol. Biochem. Behav., 79, 309-316 (2004).
S. R. Thornton and F. L. Smith, “Long-term alterations in opiate antinociception resulting from infant fentanyl tolerance and dependence,” Eur. J. Pharmacol., 363, 113-119 (1998).
H. Homayoun, S. Khavandgar, K. Namiranian, et al., “The role of nitric oxide in anticonvulsant and proconvulsant effects of morphine in mice,” Epilepsy Res., 48, 33-41 (2002).
A. I. Baamonde, A. Hidalgo, and F. Andres-Trelles, “Sex-related differences in the effects of morphine and stress on visceral pain,” Neuropharmacology, 28, 967-970 (1989).
M. Kavaliers and D. G. Innes, “Sex and day-night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis,” Pharmacol. Biochem. Behav., 27, 477-482 (1987).
I. Vathy, “Prenatal morphine exposure induces age- and sex-dependent changes in seizure susceptibility,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 25, 1203-1226 (2001).
G. Vela, J. A. Fuentes, A. Bonnin, et al., “Perinatal exposure to delta 9-tetrahydrocannabinol (delta 9-THC) leads to changes in opioid-related behavioral patterns in rats,” Brain Res., 680, 142-147 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakhjiri, E., Saboory, E., Roshan-Milani, S. et al. Prenatal Stress+Morphine and Postnatal Re-exposure to Stress Alter Pentylenetetrazol-Induced Epileptic Manifestations in Rats. Neurophysiology 48, 360–366 (2016). https://doi.org/10.1007/s11062-017-9610-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-017-9610-5