Abstract
High resolution multiphoton tomography and fluorescence lifetime imaging differentiates glioma from adjacent brain in native tissue samples ex vivo. Presently, multiphoton tomography is applied in clinical dermatology and experimentally. We here present the first application of multiphoton and fluorescence lifetime imaging for in vivo imaging on humans during a neurosurgical procedure. We used a MPTflex™ Multiphoton Laser Tomograph (JenLab, Germany). We examined cultured glioma cells in an orthotopic mouse tumor model and native human tissue samples. Finally the multiphoton tomograph was applied to provide optical biopsies during resection of a clinical case of glioblastoma. All tissues imaged by multiphoton tomography were sampled and processed for conventional histopathology. The multiphoton tomograph allowed fluorescence intensity- and fluorescence lifetime imaging with submicron spatial resolution and 200 picosecond temporal resolution. Morphological fluorescence intensity imaging and fluorescence lifetime imaging of tumor-bearing mouse brains and native human tissue samples clearly differentiated tumor and adjacent brain tissue. Intraoperative imaging was found to be technically feasible. Intraoperative image quality was comparable to ex vivo examinations. To our knowledge we here present the first intraoperative application of high resolution multiphoton tomography and fluorescence lifetime imaging of human brain tumors in situ. It allowed in vivo identification and determination of cell density of tumor tissue on a cellular and subcellular level within seconds. The technology shows the potential of rapid intraoperative identification of native glioma tissue without need for tissue processing or staining.





Similar content being viewed by others
References
Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M et al (2006) Changing paradigms—an update on the multidisciplinary management of malignant glioma. Oncologist 11:165–180
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636
Sanai N, Berger MS (2012) Recent surgical management of gliomas. Adv Exp Med Biol 746:12–25
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62:564–576
Kantelhardt SR, Diddens H, Leppert J, Rohde V, Huttmann G et al (2008) Multiphoton excitation fluorescence microscopy of 5-aminolevulinic acid induced fluorescence in experimental gliomas. Lasers Surg Med 40:273–281
Arndt-Jovin DJ, Kantelhardt SR, Caarls W, de Vries AHB, Giese A et al (2009) Tumor-targeted quantum dots can help surgeons find tumor boundaries. IEEE Trans Nanobiosci 8:65–71
Kantelhardt SR, Caarls W, de Vries AHB, Hagen GM, Jovin TM et al (2010) Specific visualization of glioma cells in living low-grade tumor tissue. PLoS ONE 5:e11323
Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M et al (2010) Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA 107:4317–4322
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S et al (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544
Smith AM, Ruan G, Rhyner MN, Nie SM (2006) Engineering luminescent quantum dots for In vivo molecular and cellular imaging. Ann Biomed Eng 34:3–14
Bohringer HJ, Boller D, Leppert J, Knopp U, Lankenau E et al (2006) Time-domain and spectral-domain optical coherence tomography in the analysis of brain tumor tissue. Lasers Surg Med 38:588–597
Bohringer HJ, Lankenau E, Stellmacher F, Reusche E, Huttmann G et al (2009) Imaging of human brain tumor tissue by near-infrared laser coherence tomography. Acta Neurochir (Wien) 151:507–517
Kantelhardt SR, Finke M, Schweikard A, Giese A (2013) Evaluation of a completely robotized neurosurgical operating microscope. Neurosurgery 72(Suppl 1):19–26
Beleites C, Geiger K, Kirsch M, Sobottka SB, Schackert G et al (2011) Raman spectroscopic grading of astrocytoma tissues: using soft reference information. Anal Bioanal Chem 400(9):2801–2816
Bergner N, Krafft C, Geiger KD, Kirsch M, Schackert G et al (2012) Unsupervised unmixing of Raman microspectroscopic images for morphochemical analysis of non-dried brain tumor specimens. Anal Bioanal Chem 403(3):719–725
Kantelhardt SR, Leppert J, Kantelhardt JW, Reusche E, Huttmann G et al (2009) Multi-photon excitation fluorescence microscopy of brain-tumour tissue and analysis of cell density. Acta Neurochir (Wien) 151:253–262
Kantelhardt SR, Leppert J, Krajewski J, Petkus N, Reusche E et al (2007) Imaging of brain and brain tumor specimens by time-resolved multiphoton excitation microscopy ex vivo. Neuro Oncol 9:103–112
Leppert J, Krajewski J, Kantelhardt SR, Schlaffer S, Petkus N et al (2006) Multiphoton excitation of autofluorescence for microscopy of glioma tissue. Neurosurgery 58:759–767
König K (2000) Multiphoton microscopy in life science. J Microsc 200:83–104
Kim D, Kim KH, Yazdanfar S, So PTC (2004) High-speed handheld multiphoton multifocimicroscopy. Proc SPIE 5323:267–272
Uchugonova A, Hoffman RM, Weinigel M, Koenig K (2011) Watching stem cells in the skin of living mice non-invasively. Cell Cycle 10(2011):2017–2020
König K, Riemann I (2003) High-resolution multiphoton tomography of human skin with subcellular spatial resolution and picoseconds time resolution. J Biomed Opt 8:432–439
Yew E, Rowlands C, So PT (2014) Application of multiphoton microscopy in dermatological studies: a mini-review. J Innov Opt Health Sci 7(5):1330010
Patalay R, Talbot C, Alexandrov Y, Lenz M, Kumar S et al (2012) Multispectral fluorescence lifetime imaging using multiphoton tomography for the evaluation of basal cell carcinomas. PLoS ONE 7(9):e43460
Balu B, Kelly K, Zachary CB, Harris RM, Krasieva TB (2014) Distinguishing between benign and malignant melanocytic nevi by in vivo multiphoton microscopy. Cancer Res 74(10):2688–2697
Palczewska G, Golczak M, Williams DR, Hunter JJ, Palczewski K (2014) Endogenous fluorophores enable two-photon imaging of the primate eye. Invest Ophthalmol Vis Sci 55(7):4438–4447
Bueno JM, Palacios R, Giakoumaki A, Gualda EJ, Schaeffel F, Artal P (2014) Retinal cell imaging in myopic chickens using adaptive optics multiphoton microscopy. Biomed Opt Express 5(3):664–674
Yan J, Zhuo S, Chen G, Milsom JW, Zhang H et al (2014) Real-time optical diagnosis for surgical margin in low rectal cancer using multiphoton microscopy. Surg Endosc 28(1):36–41
Ying M, Zhuo S, Chen G, Zhuo C, Lu J et al (2012) Real-time noninvasive optical diagnosis for colorectal cancer using multiphoton microscopy. Scanning 34(3):181–185
Sun Y, Hatami N, Yee M, Phipps J, Elson DS et al (2010) Fluorescence lifetime imaging microscopy for brain tumor image-guided surgery. J Biomed Opt 15(5):056022
Sun Y, Phipps JE, Meier J, Hatami N, Poirier B (2013) Endoscopic fluorescence lifetime imaging for in vivo intraoperative diagnosis of oral carcinoma. Microsc Microanal 19(4):791–798
Klucken J, Outeiro TF, Nguyen P, McLean PJ, Hyman BT (2006) Detection of novel intracellular alpha-synuclein oligomeric species by fluorescence lifetime imaging. FASEB J 20(12):2050–2057
Kim D, Choi H, Yazdanfar S, So PT (2008) Ultrafast optical pulse delivery with fibers for nonlinear microscopy. Microsc Res Tech 71(12):887–896
Kut C, Chaichana KL, Xi J, Raza SM, Ye X (2015) Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med 7:292ra100
Acknowledgments
Authors Karsten König and Martin Weinigel are empoyees of the Jenlab company, which produces the multiphoton microscope used in this study. However no financial support was received from this company and the company had no influence on the experiments performed. The Friedhelm-Frees-Foundation (http://www.klinik.uni-mainz.de/friedhelmfreesstiftung/) supported this study with a Grant of 10,000 Euro.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors professor Dr. Karsten König and Dr. Martin Weinigel work with the JenLab GmbH, that produces the MPT used in this study. Apart from this the authors declare that they have no conflict of interest.
Additional information
This work is part of the doctoral thesis of Darius Kalasauskas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary fig. 1—The figure shows the exact locations where the specimen shown in figs. 2A, B were taken from. A corresponds to the surface of the murine cortex, whereas B is taken from a deeper portion within the murine cortex (luxol fast blue stain (LFB) identifying myelin fibers, the white bar on the inlets A and B corresponds to a distance of 50 µm).
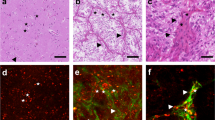
Supplementary fig. 2—Panel A shows a human transitional meningioma WHO°I and B brain metastasis of an undifferentiated adenocarcinoma of a breast cancer. The left column shows fluorescence intensity images (images marked with a1, b1; excitation 760nm), while second column (a2, b2) shows the corresponding FLIM images using a continuous color-coding (red stands for a short fluorescent lifetime of 500 ps and blue for long fluorescent lifetimes of up to 3000ps). The white bars correspond to a distance of 50 µm. The right column (a3, b3) shows the corresponding conventional histology (HE).
Rights and permissions
About this article
Cite this article
Kantelhardt, S.R., Kalasauskas, D., König, K. et al. In vivo multiphoton tomography and fluorescence lifetime imaging of human brain tumor tissue. J Neurooncol 127, 473–482 (2016). https://doi.org/10.1007/s11060-016-2062-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2062-8