Abstract
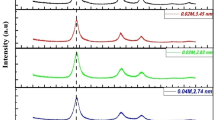
In the present study, a series of aqueous-based ZnSe(S) nanocrystals (NCs) was prepared at different solution pH ranging from 8 to 11.9, and using N-acetyl-L-cysteine (NAC) as capping agent. In addition to zinc blende structure, the X-ray diffraction studies demonstrated the quantum size regime of the ZnSe(S) NCs. To gain further insight toward the influence of the quantum confinement and pH values on optical properties of the as-prepared NCs, their UV-visible absorption and photoluminescence spectra were systematically analyzed. The absorption spectra experienced a red shift from ~340 to ~382 nm as the pH increased from 8.0 to 11.9, indicating the growth of the as-prepared ZnSe(S) NCs. The emission spectra also show the obvious red shift and the relative area of excitonic to trap emission, firstly increases from pH = 8.0 to 10.7, and then decreases by further increasing of the solution pH. The initial behavior might be due to the improved surface passivation of the trap dangling states by better deprotonation of thiol groups in NAC, whereas at pH >10.7, the faster growth rate of the ZnSe(s) NCs may lead to the formation of many defect sites. All of these phenomena were combined in the scheme which displays the effect of quantum confinement and solution pH on variation of the excitonic and trap-related emissions.

ᅟ












Similar content being viewed by others
References
Ahmad F, Pandey AK, Herzog AB, Rose JB, Gerba CP, Hashsham SA (2012) Environmental applications and potential health implications of quantum dots. J Nanopart Res 14:1038–1061
Boles MA, Ling D, Hyeon T, Talapin DV (2016) The surface science of nanocrystals. Nat Mater 15:141–153
Brus L (1986) Electronic wave functions in semiconductor clusters: experiment and theory. J Phys Chem 90:2555–2560
Cao J, Xue B, Li H, Deng D, Gu Y (2010) Facile synthesis of high-quality water-soluble N-acetyl-l-cysteine-capped Zn1−xCdxSe/ZnS core/shell quantum dots emitting in the violet–green spectral range. J Colloid Interface Sci 348:369–376
Choi J, Yoon S, Kim FS, Kim N (2016) Aqueous-phase synthesis and color-tuning of core/shell/shell inorganic nanocrystals consisting of ZnSe,(Cu, Mn)-doped ZnS, and ZnS. J Alloys Compd 671:360–365
Choy WC, Xiong S, Sun Y (2009) A facile synthesis of zinc blende ZnSe nanocrystals. J Phys D Appl Phys 42:125410
Cui L, He XP, Chen G-R (2015) Recent progress in quantum dot based sensors. RSC Adv 5:26644–26653
Dean JA (1968) Lange’s handbook of chemistry. McGrawHill, Inc., Knoxville, TN
Deng Z, Lie FL, Shen S, Ghosh I, Mansuripur M, Muscat AJ (2009) Water-based route to ligand-selective synthesis of ZnSe and Cd-doped ZnSe quantum dots with tunable ultraviolet A to blue photoluminescence. Langmuir 25:434–442
Diestra DD, Huarac JB, Rinco DPB, Feliciano JAG, Gonzalez CI, Weiner BR, Morell G (2015) Biocompatible ZnS:Mn quantum dots for reactive oxygen generation and detection in aqueous media. J Nanopart Res 17:461–474
Duan J, Jiang X, Ni S, Yang M, Zhan J (2011) Facile synthesis of N-acetyl-L-cysteine capped ZnS quantum dots as an eco-friendly fluorescence sensor for Hg2+. Talanta 85:1738–1743
Dunstan D, Nicholls J, Cavenett B, Davies J (1980) Zinc vacancy-associated defects and donor-acceptor recombination in ZnSe. J Phys C Solid State 13:6409
Efros AL (2008) Nanocrystals: almost always bright. Nat Mater 7:612–613
Gaponik N et al (2002) Thiol-capping of CdTe nanocrystals: an alternative to organometallic synthetic routes. J Phys Chem B 106:7177–7185
Hu Z, Xu S, Xu X, Wang Z, Wang Z, Wang C, Cui Y (2015) Co-doping of Ag into Mn:ZnSe quantum dots: giving optical filtering effect with improved monochromaticity. Sci Rep 5:14817
Jeon DY, Gislason H, Watkins GD (1993) Optical detection of magnetic resonance of the zinc vacancy in ZnSe via magnetic circular dichroism. Phys Rev B 48:7872
Kho R, Martinez CLT, Mehra RK (2000) A simple colloidal synthesis for gram-quantity production of water-soluble ZnS nanocrystal powders. J Colloid Interface Sci 227:561–566
Kiplagat A, Sibuyi NR, Onani MO, Meyer M, Madiehe AM (2016) The cytotoxicity studies of water-soluble InP/ZnSe quantum dots. J Nanopart Res 18:147–158
Klimov VI, McBranch D, Leatherdale C, Bawendi M (1999) Electron and hole relaxation pathways in semiconductor quantum dots. Phys Rev B 60:13740
Konstantatos G, Sargent EH (2013) Colloidal quantum dot optoelectronics and photovoltaics. Cambridge University Press, New York
Lan GY, Lin YW, Lin ZH, Chang HT (2010) Synthesis and characterization of ZnxHg1− xSeyS1− y quantum dots. J Nanopart Res 12:1377–1388
Lee YS, Bu HB, Taniguchi T, Takagi T, Sobue S, Yamada H, Iwaki T, Kim D (2016) Hydrothermal synthesis of NAC-capped II-VI semiconductor ZnSe quantum dots in acidic condition. Chem Lett 45:878–880
Lesnyak V, Gaponik N, Eychmüller A (2013) Colloidal semiconductor nanocrystals: the aqueous approach. Chem Soc Rev 42:2905–2929
Li LS, Pradhan N, Wang Y, Peng X (2004) High quality ZnSe and ZnS nanocrystals formed by activating zinc carboxylate precursors. Nano Lett 4:2261–2264
Li C, Nishikawa K, Ando M, Enomoto H, Murase N (2008) Synthesis of Cd-free water-soluble ZnSe1−xTex nanocrystals with high luminescence in the blue region. J Colloid Interface Sci 321:468–476
Liu FC, Cheng TL, Shen CC, Tseng WL, Chiang MY (2008) Synthesis of cysteine-capped ZnxCd1-xSe alloyed quantum dots emitting in the blue-green spectral range. Langmuir 24:2162–2167
Luong BT, Hyeong E, Yoon S, Choi J, Kim N (2013) Facile synthesis of UV-white light emission ZnSe/ZnS: Mn core/(doped) shell nanocrystals in aqueous phase. RSC Adv 3:23395–23401
Mahler B, Spinicelli P, Buil S, Quelin X, Hermier JP, Dubertret B (2008) Towards non-blinking colloidal quantum dots. Nat Mater 7:659–664
Molaei M, Bahador A, Karimipour M (2015) Green synthesis of ZnSe and core–shell ZnSe-ZnS nanocrystals (NCs) using a new, rapid and room temperature photochemical approach. J Lumin 166:101–105
Osipovich NP, Shavel A, Poznyak SK, Gaponik N, Eychmüller A (2006) Electrochemical observation of the photoinduced formation of alloyed ZnSe(S) nanocrystals. J Phys Chem B 110:19233–19237
Parak WJ, Manna L, Simmel FC, Gerion D, Alivisatos P (2004) Quantum dots nanoparticles: from theory to application: 4–49. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Peng X (2009) An essay on synthetic chemistry of colloidal nanocrystals. Nano Res 2:425–447
Peng X, Manna L, Yang W, Wickham J, Scher E, Kadavanich A, Alivisatos AP (2000) Shape control of CdSe nanocrystals. Nature 404:59–61
Piven N, Susha AS, Döblinger M, Rogach AL (2008) Aqueous synthesis of alloyed CdSexTe1-x nanocrystals. J Phys Chem C 112:15253–15259
Pradhan N, Goorskey D, Thessing J, Peng X (2005) An alternative of CdSe nanocrystal emitters: pure and tunable impurity emissions in ZnSe nanocrystals. J Am Chem Soc 127:17586–17587
Qian H, Qiu X, Li L, Ren J (2006) Microwave-assisted aqueous synthesis: a rapid approach to prepare highly luminescent ZnSe(S) alloyed quantum dots. J Phys Chem B 110:9034–9040
Qin H, Jian W, Zhang Y, Kim T, Jiang Z, Jiang D, Sun D (2012) A simple and novel route for the synthesis of water soluble ZnSe quantum dots using the nano-Se as the reaction intermediate. Mater Lett 67:28–31
Regulacio MD, Han MY (2010) Composition-tunable alloyed semiconductor nanocrystals. Accounts Chem Res 43:621–630
Reiss P (2007) ZnSe based colloidal nanocrystals: synthesis, shape control, core/shell, alloy and doped systems. New J Chem 31:1843–1852
Saikia K, Deb P, Kalita E (2013) Sensitive fluorescence response of ZnSe(S) quantum dots: an efficient fluorescence probe. Phys Scr 87:065802
Sapra S, Sarma D (2004) Evolution of the electronic structure with size in II-VI semiconductor nanocrystals. Phys Rev B 69:125304
Shavel A, Gaponik N, Eychmüller A (2004) Efficient UV-blue photoluminescing thiol-stabilized water-soluble alloyed ZnSe(S) nanocrystals. J Phys Chem B 108:5905–5908
Shavel A, Gaponik N, Eychmüller A (2006) Factors governing the quality of aqueous CdTe nanocrystals: calculations and experiment. J Phys Chem B 110:19280–19284
Shen CC, Tseng WL (2009) One-step synthesis of white-light-emitting quantum dots at low temperature. Inorg Chem 48:8689–8694
Shirakawa Y, Kukimoto H (1980) The electron trap associated with an anion vacancy in ZnSe and ZnSxSe1− x. Solid State Commun 34:359–361
Soheyli E, Sahraei R, Nabiyouni G (2016) Aqueous based synthesis of N-acetyl-l-cysteine capped ZnSe nanocrystals with intense blue emission. Opt Mater 60:564–570
Srivastava BB, Jana S, Karan NS, Paria S, Jana NR, Sarma D, Pradhan N (2010) Highly luminescent Mn-doped ZnS nanocrystals: gram-scale synthesis. J Phys Chem Lett 1:1454–1458
Srivastava P, Kumar P, Singh K (2011) Room temperature ferromagnetism in magic-sized Cr-doped CdS diluted magnetic semiconducting quantum dots. J Nanopart Res 13:5077–5085
Suganthi ARB, Joshi AG, Sagayaraj P (2012) A novel two-phase thermal approach for synthesizing CdSe/CdS core/shell nanostructure. J Nanopart Res 14:691–699
Sugimoto T (1987) Preparation of monodispersed colloidal particles. Adv Colloid Interf Sci 28:65–108
Talapin DV, Lee JS, Kovalenko MV, Shevchenko EV (2009) Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem Rev 110:389–458
Viol LCS, Raphael E, Bettini J, Ferrari JL, Schiavon MA (2014) A simple strategy to prepare colloidal Cu-doped ZnSe(S) green emitter nanocrystals in aqueous media. Part Part Syst Charact 31:1084–1090
Viswanatha R, Brovelli S, Pandey A, Crooker SA, Klimov VI (2011) Copper-doped inverted core/shell nanocrystals with “permanent” optically active holes. Nano Lett 11:4753–4758
Wang C, Xu S, Wang Y, Wang Z, Cui Y (2014) Aqueous synthesis of multilayer Mn: ZnSe/Cu: ZnS quantum dots with white light emission. J Mater Chem C 2:660–666
Wang Y, Wu B, Yang C, Liu M, Sum TC, Yong KT (2016) Synthesis and characterization of Mn: ZnSe/ZnS/ZnMnS sandwiched QDs for multimodal imaging and theranostic applications. Small 12:534–546
Wuister SF, Donega CM, Meijerink A (2004) Influence of thiol capping on the exciton luminescence and decay kinetics of CdTe and CdSe quantum dots. J Phys Chem B 108:17393–17397
Xiao Q, Huang S, Su W, Chan WH, Liu Y (2012) Facile synthesis and characterization of highly fluorescent and biocompatible N-acetyl-L-cysteine capped CdTe/CdS/ZnS core/shell/shell quantum dots in aqueous phase. Nanotechnology 23:495717
Xu S, Wang C, Zhang H, Wang Z, Yang B, Cui Y (2011) pH-sensitive photoluminescence for aqueous thiol-capped CdTe nanocrystals. Nanotechnology 22:315703
Yang F, Xu Z, Wang J, Zan F, Dong C, Ren J (2013) Microwave-assisted aqueous synthesis of new quaternary-alloyed CdSeTeS quantum dots and their bioapplications in targeted imaging of cancer cells. Lumin 28:392–400
Zan F, Ren J (2010) Significant improvement in photoluminescence of ZnSe(S) alloyed quantum dots prepared in high pH solution. Lumin 25:378–383
Zeng R, Shen R, Zhao Y, Li X, Sun Z, Shen Y (2014) Aqueous synthesis of Cu-doped ZnCdS/ZnS core/shell nanocrystals with a new and highly reactive sulfur source. Nanotechnology 25:135602
Zhang H, Wang D, Yang B, Möhwald H (2006) Manipulation of aqueous growth of CdTe nanocrystals to fabricate colloidally stable one-dimensional nanostructures. J Am Chem Soc 128:10171–10180
Zhang H, Liu Y, Wang C, Zhang J, Sun H, Li M, Yang B (2008a) Directing the growth of semiconductor nanocrystals in aqueous solution: role of electrostatics. Chem Phys Chem 9:1309–1316
Zhang H, Liu Y, Zhang J, Wang C, Li M, Yang B (2008b) Influence of interparticle electrostatic repulsion in the initial stage of aqueous semiconductor nanocrystal growth. J Phys Chem C 112:1885–1889
Zhang J, Li J, Zhang J, Xie R, Yang W (2010) Aqueous synthesis of ZnSe nanocrystals by using glutathione as ligand: the pH-mediated coordination of Zn2+ with glutathione. J Phys Chem C 114:11087–11091
Zhao D, He Z, Chan W, Choi MM (2008) Synthesis and characterization of high-quality water-soluble near-infrared-emitting CdTe/CdS quantum dots capped by N-acetyl-L-cysteine via hydrothermal method. J Phys Chem C 113:1293–1300
Zhao D, Fang Y, Wang H, He Z (2011) Synthesis and characterization of high-quality water-soluble CdTe: Zn2+ quantum dots capped by N-acetyl-L-cysteine via hydrothermal method. J Mater Chem 21:13365–13370
Zhao D, Li JT, Gao F, Cl Z, He ZK (2014) Facile synthesis and characterization of highly luminescent UV-blue-emitting ZnSe/ZnS quantum dots via a one-step hydrothermal method. RSC Adv 4:47005–47011
Acknowledgement
E. Soheyli likes to thank Mr. Ehsan Smailpour for his assistance in drawing the suggested schemes. The authors also thank Dr. Dan Zhao for his valuable suggestions over the precipitation method.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 5471 kb).
Rights and permissions
About this article
Cite this article
Soheyli, E., Sahraei, R. & Nabiyouni, G. pH-dependent optical properties of N-acetyl-L-cysteine-capped ZnSe(S) nanocrystals with intense/stable emissions. J Nanopart Res 19, 92 (2017). https://doi.org/10.1007/s11051-017-3792-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3792-z