Abstract
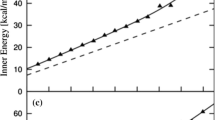
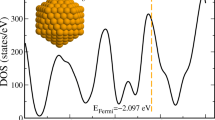
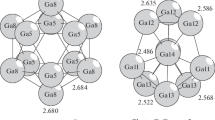
Theoretical insights are provided for understanding why the structural changes detected by photoelectronic measurements on the canonical ensemble of clusters Na\(_{309}\) do not induce any signal on their experimental specific heat. The cooling down of a collection of these clusters, whose atomic interactions are modeled with the Gupta potential, reveals that Na\(_{309}\) has indeed two configurations of enhanced stability. The most stable one is a perfect icosahedral structure (ico configuration), just as was determined by experiments. The candidate derived from the interaction model for the unknown configuration is a kind of hcp variation of the first (ico-hcp configuration). It has more atoms with low coordination, but also more atoms with high coordination. But the difference between the quantities of the first ones is greater than the difference between the amounts of the second ones, causing a slight loss of the cluster cohesion. Additionally, the properties of an ensemble of Na\(_{309}\) clusters in thermodynamic equilibrium were determined. The comparison between theoretical and experimental results shows that the model only reproduces the properties of the solid–solid transition. However, its predictions for the melting and premelting are wrong. We suggest that both failures could be consequences of only one elementary fault: the underestimation of the cohesion of the atoms with low coordination. This source of error of the model would not affect the description of the structural transition, since this transformation is essentially caused by clusters made up of atoms with high coordination. In spite of its mistakes, the model allowed to determine for the first time a candidate for the unknown solid phase of these clusters. This structure, as well as the elementary fault suggested for the model, must be confirmed by ab-initio calculations.





Similar content being viewed by others
References
Aguado A, Jarrold MF (2011) Melting and freezing of metal clusters. Annu Rev Phys Chem 62:151–172
Aguado A, López JM (2005) Anomalous size dependence in the melting temperatures of free sodium clusters: an explanation for the calorimetry experiments. Phys Rev Lett 94(23):233401
Bixon M, Jortner J (1989) Energetic and thermodynamic size effects in molecular clusters. J Chem Phys 91:1631–1642
Breaux GA, Benirschke RC, Sugai T, Kinnear BS, Jarrold MF (2003) Hot and solid gallium clusters: too small to melt. Phys Rev Lett 91(21):215508
Breaux GA, Neal CM, Cao B, Jarrold MF (2005) Melting, premelting, and structural transitions in size- selected aluminum clusters with around 55 atoms. Phys Rev Lett 94(17):173401
Calvo F, Labastie P (1995) Configurational density of states from molecular dynamics simulations. Chem Phys Lett 247:395–400
Cleveland CL, Luedtke WD, Landman U (1998) Melting of gold clusters: icosahedral precursors. Phys Rev Lett 81:2036–2039
Cleveland CL, Luedtke WD, Landman U (1999) Melting of gold clusters. Phys Rev B 60:5065–5077
Gupta RP (1981) Lattice relaxation at a metal surface. Phys Rev B 23:6265–6270
Haberland H, Hippler T, Donges J, Kostko O, Schmidt M, von Issendorff B (2005) Melting of sodium clusters: where do the magic numbers come from? Phys Rev Lett 94(3):035701
Hvolbæk B, Janssens TVW, Clausen BS, Falsig H, Christensen CH, Nørskov JK (2007) Catalytic activity of Au nanoparticles. Nanotoday 2:14–18
Jellinek J, Beck TL, Berry RS (1986) Solid–liquid phase changes in simulated isoenergetic Ar\(_{13}\). J Chem Phys 84:2783–2794
Koga K, Ikeshoji T, Sugawara KI (2004) Size- and temperature- dependent structural transitions in gold nanoparticles. Phys Rev Lett 92(11):115507
Kostko O, Huber B, Moseler M, von Issendorff B (2007) Structure determination of medium- sized sodium clusters. Phys Rev Lett 98(4):043401
Labastie P, Whetten RL (1990) Statistical thermodynamics of the cluster solid–liquid transition. Phys Rev Lett 65:1567–1570
Li Y, Blaisten-Barojas E, Papaconstantopoulos DA (1998) Structure and dynamics of alkali-metal clusters and fission of highly charged clusters. Phys Rev B 57:15519–15532
Noya EG, Doye JPK, Calvo F (2006) Theoretical study of the melting of aluminum clusters. Phys Rev B 73(12):125407
Noya EG, Doye JPK, Wales DJ, Aguado A (2007) Geometric magic numbers of sodium clusters: interpretation of the melting behaviour. Eur Phys J D 43:57–60
Plant SR, Cao L, Palmer RE (2014) Atomic structure control of size—selected gold nanoclusters during formation. J Am Chem Soc 136:7559–7562
Reyes-Nava JA, Garzón IL, Michaelian K (2003) Negative heat capacity of sodium clusters. Phys Rev B 67(16):165401
Schmidt M, Kusche R, Kronmüller W, von Issendorff B, Haberland H (1997) Experimental determination of the melting point and heat capacity for a free cluster of 139 sodium atoms. Phys Rev Lett 79:99–102
Schmidt M, Kusche R, von Issendorff B, Haberland H (1998) Irregular variations in the melting point of size-selected atomic clusters. Nature 393:238–240
Schmidt M, Kusche R, Hippler T, Donges J, Kronmüller W, von Issendorff B, Haberland H (2001) Negative heat capacity for a cluster of 147 sodium atoms. Phys Rev Lett 86:1191–1194
Starace AK, Cao B, Judd OH, Bhattacharyya I, Jarrold MF (2010) Melting of size-selected aluminum nanoclusters with 84–28 atoms. J Chem Phys 132(3):034302
Wang ZW, Palmer RE (2012) Determination of the ground- state atomic structures of size- selected Au nanoclusters by electron- beam- induced transformation. Phys Rev Lett 108(24):245502
Acknowledgments
This work was supported by Promep-México under the Project grant UNICACH/103.5/12/3513. J.A.R.N. thanks Professor Martin Schmidt (Laboratoire Aimé Cotton, France) for fruitful discussions, computational supports from Laboratorio de Supercómputo del Centro de Investigación y Desarrollo Tecnológico en Energías Renovables (LSC-CIDTER-UNICACH, México), DGTIC-UNAM project No. SC16-1-IR-113 for cpu-time, and ABACUS-CINVESTAV under CONACYT grant EDOMEX-2011-COI-165873 also for cpu-time. I.L.G. acknowledges support from Conacyt-México under Project 177981.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reyes-Nava, J.A., Moreira, J., Pantoja, J. et al. The structural transition of the Na\(_{309}\) clusters. J Nanopart Res 18, 276 (2016). https://doi.org/10.1007/s11051-016-3558-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3558-z