Abstract
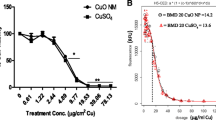
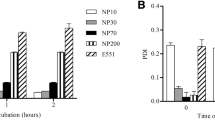
Different concentrations of CuSO4, micro-CuO, and nano-CuO were added to Caco-2 cell monolayers to study the absorption and transport characteristics in this epithelial cell model. Nano-CuO nanoparticles had a diameter of 10–20 nm. Inhibitors of endocytosis were used to explore whether nano-CuO could enter the Caco-2 cell in the form of nanoparticles, and to ascertain the endocytotic pathway that is involved in the transport process. The apparent permeability coefficient (P app) of CuSO4 and nano-CuO increased with the Cu concentration in the culture medium (p < 0.05). The micro-CuO of different concentrations had no significant impact on the P app value of Caco-2 cells (p > 0.05). When the Cu concentration in the culture medium was in the range 31.25–500 μM, the P app value of Caco-2 cells incubated with nano-CuO was significantly higher than that obtained with CuSO4. The latter was also significantly higher than that when cells were incubated with micro-CuO (p < 0.05). The amount of Cu transport increased with the increase of CuSO4 concentration in the culture medium. After 90 min, the amount of transport began to saturate, and the transport rate of Cu declined with the increase of CuSO4 concentration. For the cells incubated with nano-CuO, the amount of Cu transport increased with the increase of nano-CuO concentration, but did not show an obvious saturation with the extension of transport time. Nano-CuO could enter the Caco-2 cell in the form of nanoparticles, and were found in the cytoplasm, vesicles, lysosomes, and cell nuclei. Several inhibitors of endocytosis effectively prevented the entry of nano-CuO into the Caco-2 cells. It was concluded that nano-CuO particles can enter the Caco-2 cells through several cellular endocytotic pathways.








Similar content being viewed by others
References
Bauerly KA, Kelleher SL, Lönnerdal B (2004) Functional and molecular responses of suckling rat pups and human intestinal Caco-2 cells to copper treatment. J Nutr Biochem 15(3):155–162
Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422:37–44
Goldstein JL, Anderson RG, Brown MS (1979) Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 279:679–685
Gu J, Xu H, Han Y, Dai W, Hao W, Wang C, Gu N, Xu H, Cao J (2011) The internalization pathway, metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cell. Sci China Life Sci 54(9):793–805
Harush-Frenkel O, Debotton N, Benita S, Altschuler Y (2007) Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun 353:26–32
Harush-Frenkel O, Altschuler Y, Benita S (2008) Nanoparticle–cell interactions: drug delivery implications. Crit Rev Ther Drug Carr Syst 25(6):485–544
Jani P, Halbert GW, Langridge J, Florence AT (1990) Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol 42(12):821–826
Jones AT (2007) Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med 11:670–684
Kaksonen M, Toret CP, Drubin DG (2006) Harnessing actin dynamics for clathrin mediated endocytosis. Nat Rev Mol Cell Biol 7:404–414
Kam NW, Liu Z, Dai H (2006) Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew Chem Int Ed Engl 45:577–581
Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S, Luangsivilay J, Godefroy S, Pantarotto D, Briand JP, Muller S, Prato M, Bianco A (2007) Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol 2:108–113
Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M (2003) Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci USA 100:13964–13969
Lajoie P, Nabi IR (2007) Regulation of raft-dependent endocytosis. Cell Mol Med 11:644–653
Merrifield CJ (2004) Seeing is believing: imaging actin dynamics at single sites of endocytosis. Trends Cell Biol 14:352–358
Park JS, Han TH, Lee KY, Han SS, Hwang JJ, Moon DH, Kim SY, Cho YW (2006) N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: endocytosis, exocytosis and drug release. J Control Release 115:37–45
Piret JP, Vankoningsloo S, Mejia J, Noël F, Boilan E, Lambinon F, Zouboulis CC, Masereel B, Lucas S, Saout C, Toussaint O (2012) Differential toxicity of copper(II) oxide nanoparticles of similar hydrodynamic diameter on human differentiated intestinal Caco-2 cell monolayers is correlated in part to copper release and shape. Nanotoxicology 6(7):789–803
Wang LH, Rothberg KG, Anderson RG (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. Cell Biol 123:1107–1117
West MA, Bretscher MS, Watts C (1989) Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol 109:2731–2739
Yee SY (1997) In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm Res 14(6):763–766
Acknowledgments
This article was supported by National Natural Science Foundation of China (31372498), Directors’ Foundation of the National Natural Science Foundation of China (31140076), and Basic Projects of Qingdao Science Planning Program (12-1-4-5-(5)-jch).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Lianqin, Z., Fenghua, Z. et al. Comparative evaluation of nano-CuO crossing Caco-2 cell monolayers and cellular uptake. J Nanopart Res 17, 195 (2015). https://doi.org/10.1007/s11051-015-3005-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3005-6