Abstract
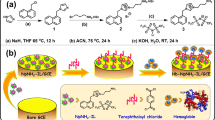
Human hemoglobin is an important metalloprotein. It has tetrameric structure with each subunit containing a ‘heme’ group which carries oxygen and carbon dioxide in blood. In this work, we have investigated the interactions of human hemoglobin (Hb) with charged ligand-functionalized iron oxide nanoparticles and the effect of counterions, in aqueous medium. Several techniques like DLS and ζ-potential measurements, UV–vis, fluorescence, and CD spectroscopy have been used to characterize the interaction. The nanoparticle size was measured to be in the range of 20–30 nm. Our results indicated the binding of Hb with both positively as well as negatively charged ligand-functionalized iron oxide nanoparticles in neutral aqueous medium which was driven by the electrostatic and the hydrophobic interactions. The electrostatic binding interaction was not seen in phosphate buffer at pH 7.4. We have also observed that the ‘heme’ groups of Hb remained unaffected on binding with charged nanoparticles, suggesting the utility of the charged ligand-functionalized nanoparticles in biomedical applications.





Similar content being viewed by others
References
Bajaj A, Miranda OR, Kim IB, Phillips RL, Jerry DJ, Bunz UHF, Rotello VM (2009) Detection and differentiation of normal, cancerous, and metastatic cells using nanoparticle-polymer sensor arrays. Proc Natl Acad Sci USA 106:10912–10916
Cao YC (2008) Nanomaterials for biomedical applications. Nanomedicine 3:467–469
Chen K, Xu Y, Rana S, Miranda OR, Dubin PL, Rotello VM, Sun L, Guo X (2011) Electrostatic selectivity in protein-nanoparticle interactions. Biomacromolecules 12:2552–2561
De M, Ghosh PS, Rotello VM (2008) Applications of nanoparticles in biology. Adv Mater 20:4225–4241
Fang C, Zhang M (2009) Multifunctional magnetic nanoparticles for medical imaging applications. J Mater Chem 19:6258–6266
Garabagiu S (2011) A spectroscopic study on the interaction between gold nanoparticles and hemoglobin. Mater Res Bull 46:2474–2477
Ghosh G, Panicker L, Ningthoujam RS, Barick KC, Tewari R (2013) Counter ion induced irreversible denaturation of hen egg white lysozyme upon electrostatic interaction with iron oxide nanoparticles: a predicted model. Colloids Surf B 103:267–274
Ghosh G, Panicker L, Barick KC (2014a) Selective binding of proteins on functional nanoparticles via reverse charge parity model: an in vitro study. Mater Res Express 1(1–7):015017
Ghosh G, Panicker L, Barick KC (2014b) Protein nanoparticle electrostatic interaction: size dependent counterions induced conformational change of hen egg white lysozyme. Colloids Surf B 118:1–6
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Laurent S, Forge D, Port M, Roch A, Robic C, Elst IV, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064–2110
Linse S, Cabaleiro-Lago C, Xue WF, Lynch I, Lindman S, Thulin E, Radford SE, Dawson KA (2007) Nucleation of protein fibrillation by nanoparticles. Proc Natl Acad Sci USA 104:8691–8696
Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA (2007) The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci 134–135:167–174
Mahato M, Pal P, Tah B, Ghosh M, Talapatro GB (2011) Study of silver nanoparticle-hemoglobin interaction and composite formation. Colloids Surf B 88:141–149
Milani S, Bombelli FB, Pitek AS, Dawson KA, Rädler J (2012) Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard corona. ACS Nano 6:2532–2541
Miranda OR, You CC, Philips R, Kim IB, Ghosh PS, Bunz UHF, Rotello VM (2007) Array-based sensing of proteins using conjugated polymers. J Am Chem Soc 129:9856–9857
Monopoli MP, Åberg C, Salvati A, Dawson KA (2012) Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 7:779–786
Neely A, Perry C, Varisli B, Singh AK, Arbneshi T, Senapati D, Kalluri JR, Ray PC (2009) Ultrasensitive and highly selective detection of Alzheimer’s disease biomarker using two-photon Rayleigh scattering properties of gold nanoparticle. ACS Nano 3:2834–2840
Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8:543–557
Pankhurst QA, Connolly J, Jones SK, Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36:R167–R181
Phillips RL, Miranda OR, You CC, Rotello VM, Bunz UHF (2008) Rapid and efficient identification of bacteria using gold-nanoparticle-poly(para-phenyleneethynylene) constructs. Angew Chem Int Ed 7:2590–2594
Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA (2013) Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol 8:137–143
Sengupta B, Sengupta PK (2002) The interaction of Quercetin with Human Serum Albumin: a fluorescence spectroscopic study. Biochem Biophys Res Commun 299:400–403
Shang W, Nuffer JH, Muniz-Papandrea VA, Colon W, Siegel RW, Dordick JS (2009) Cytochrome C on silica nanoparticles: influence of nanoparticle size on protein structure, stability, and activity. Small 5:470–476
Sokolov L, Mukherji I (2000) Structure of sickle cell hemoglobin fibers probed with UV Resonance Raman spectroscopy. J Phys Chem B 104:10835–10843
Sun W, Wang D, Zhai Z, Gao R, Jiao K (2009) Direct electrochemistry of hemoglobin immobilized in the sodium alginate and SiO2 nanoparticles biocomposite film on a carbon ionic liquid electrode. J Iran Chem Soc 6:412–419
Wang B, Zhang L, Bae SC, Granick S (2008) Nanoparticle-induced surface reconstruction of phospholipid membranes. Proc Natl Acad Sci USA 105:18171–18175
Wang YQ, Zhang HM, Zhou QH, Xu HL (2009) A study of the binding of colloidal Fe3O4 with bovine hemoglobin using optical spectroscopy. Colloids Surf A 337:102–108
Xu Y, Zheng Y, Fan JS, Yang D (2006) A new strategy for structure determination of large proteins in solution without deuteration. Nat Methods 3:931–937
Zolghadri S, Saboury AA, Amin E, Moosavi-Movahedi AA (2010) A spectroscopic study on the interaction between ferric oxide nanoparticles and human hemoglobin. J Iran Chem Soc 7:S145–S153
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghosh, G., Panicker, L. Interactions of human hemoglobin with charged ligand-functionalized iron oxide nanoparticles and effect of counterions. J Nanopart Res 16, 2800 (2014). https://doi.org/10.1007/s11051-014-2800-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2800-9