Abstract
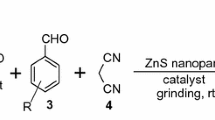
An efficient and green protocol for the synthesis of 1-substituted-1H-tetrazoles through cyclization reaction of various primary amines, sodium azide, and triethyl orthoformate was described. In this method, a series of tetrazole derivatives was synthesized by using ZnS nanoparticles as an effective, recoverable, and reusable catalyst under solvent-free conditions. This strategy is a magnificent improvement for the synthesis of these heterocycles due to the non-acidic, clean, and solvent-free conditions via a solid recyclable catalyst. The catalyst was separated by simple filtration and reused seven times without significant loss of activity. The ZnS nanoparticles with high surface area and fine monodisperse particles were prepared using the simple microwave-assisted method without using any surfactant. The ZnS nanoparticle catalyst is a good candidate to replace brønsted acids and metal salts or other catalyst for the preparation of 1-substituted-1H-tetrazoles in high yields and has potential values for industrial applications.
Graphical Abstract









Similar content being viewed by others
References
Burger A (1991) Isosterism and bioisosterism in drug design. Prog Drug Res 37:287–371
Butler RN (1996) Tetrazoles. In: Alan RK, Charles WR, Scriven EFV (eds) Comprehensive heterocyclic chemistry II. Pergamon, Oxford, pp 621–678
Curran DP, Hadida S, Kim SY (1999) Tris-(2-perfluorohexylethyl) tin azide: a new reagent for preparation of 5-substituted tetrazoles from nitriles with purification by fluorous/organic liquid–liquid extraction. Tetrahedron 55(29):8997–9006
Dighe SN, Jain KS, Srinivasan KV (2009) A novel synthesis of 1-aryl tetrazoles promoted by employing the synergy of the combined use of DMSO and an ionic liquid as the solvent system at ambient temperature. Tetrahedron Lett 50(45):6139–6142
Ebrahimizadeh Abrishami M, Kompany A, Hosseini SM, Ghajari Bardar N (2012) Preparing undoped and Mn-doped ZnO nanoparticles: a comparison between sol–gel and gel-combustion methods. J Sol–Gel Sci Technol 62(2):153–159
Goossen LJ, Melzer B (2007) Synthesis of valsartan via decarboxylative biaryl coupling. J Org Chem 72(19):7473–7476
Gupta AK, Song CH, Oh CH (2004) 1-(2-Iodophenyl)-1H-tetrazole as a ligand for Pd (II) catalyzed Heck reaction. Tetrahedron Lett 45(21):4113–4116
Huynh MHV, Hiskey MA, Chavez DE, Naud DL, Gilardi RD (2005) Synthesis, characterization, and energetic properties of diazido heteroaromatic high-nitrogen CN compound. J Am Chem Soc 127(36):12537–12543
Jin T, Kitahara F, Kamijo S, Yamamoto Y (2008) Copper-catalyzed synthesis of 5-substituted 1-H-tetrazoles via the [3 + 2] cycloaddition of nitriles and trimethylsilyl azide. Tetrahedron Lett 49(17):2824–2827
Kantam ML, Kumar SKB, K RP (2006) An efficient synthesis of 5-substituted 1-H-tetrazoles using Zn/Al hydrotalcite catalyst. J Mol Catal A 247(1):186–188
Khalafi-Nezhad A, Mohammadi S (2013) Highly efficient synthesis of 1- and 5-substituted 1H-tetrazoles using chitosan derived magnetic ionic liquid as a recyclable biopolymer-supported catalyst. RSC Adv 3(13):4362–4371. doi:10.1039/C3RA23107K
Khorsand Zak A, Majid WHA, Ebrahimizadeh Abrishami M, Yousefi R, Parvizi R (2012) Synthesis, magnetic properties and X-ray analysis of Zn0.97X0.03O nanoparticles (X = Mn, Ni, and Co) using Scherrer and size–strain plot methods. Solid State Sci 14(4):488–494
Kundu D, Majee A, Hajra A (2009) Indium triflate-catalyzed one-pot synthesis of 1-substituted-1H-1,2,3,4-tetrazoles under solvent-free conditions. Tetrahedron Lett 50(22):2668–2670
Lang L, Li B, Liu W, Jiang L, Xu Z, Yin G (2010) Mesoporous ZnS nanospheres: a high activity heterogeneous catalyst for synthesis of 5-substituted 1H-tetrazoles from nitriles and sodium azide. Chem Commun 46(3):448–450. doi:10.1039/B912284B
Lang L, Zhou H, Xue M, Wang X, Xu Z (2013) Mesoporous ZnS hollow spheres-catalyzed synthesis of 5-substituted 1H-tetrazoles. Mater Lett 106:443–446
Muraglia E, Kinzel OD, Laufer R, Miller MD, Moyer G, Munshi V, Orvieto F, Palumbi MC, Pescatore G, Rowley M (2006) Tetrazole thioacetanilides: potent non-nucleoside inhibitors of WT HIV reverse transcriptase and its K103 N mutant. Bioorg Med Chem Lett 16(10):2748–2752
Nasrollahzadeh M, Bayat Y, Habibi D, Moshaee S (2009) FeCl3-SiO2 as a reusable heterogeneous catalyst for the synthesis of 5-substituted 1-H-tetrazoles via [2 + 3] cycloaddition of nitriles and sodium azide. Tetrahedron Lett 50(31):4435–4438
Popova EA, Trifonov RE, Ostrovskii VA (2012) Advances in the synthesis of tetrazoles coordinated to metal ions. Arkivoc 1:45–65
Potewar TM, Siddiqui SA, Lahoti RJ, Srinivasan KV (2007) Efficient and rapid synthesis of 1-substituted 1H-1,2,3,4-tetrazoles in the acidic ionic liquid 1-N-butylimidazolium tetrafluoroborate. Tetrahedron Lett 48(10):1721–1724
Qiao LG, Asif A, Shi WF (2011) Synthesis and shape memory behavior study of hyperbranched poly (urethane-tetrazole). Sci China Chem 54(9):1461–1467
Santen RA, Neurock M (2006) Molecular heterogeneous catalysis: a conceptual and computational approach. Wiley, VHC, Germany
Singh H, Chawla AS, Kapoor VK, Paul D, Malhotra RK (1980) Medicinal chemistry of tetrazoles. Prog Med Chem 17:151–183
Su WK, Hong Z, Shan WG, Zhang XX (2006) A facile synthesis of 1-substituted-1H-1,2,3,4-tetrazoles catalyzed by ytterbium triflate hydrate. Eur J Org Chem 12:2723–2726
Suresh S (2013) Synthesis, structural and dielectric properties of zinc sulfide nanoparticles. Int J Phys Sci 8(21):1121–1127
Wang M, Sun L, Fu X, Liao C, Yan C (2000) Synthesis and optical properties of ZnS:Cu(II) nanoparticles. Solid State Commun 115(9):493–496
Wittenberger SJ (1994) Recent developments in tetrazole chemistry. A review. Org Prep Proced Int 26(5):499–531
Zhang C, Zheng G, Fang L, Li Y (2006) Efficient synthesis of valsartan, a nonpeptide angiotensin II receptor antagonist. Synlett 17(3):475–477
Acknowledgments
The authors are grateful to University of Kashan for supporting this work by Grant No. 159148/25.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naeimi, H., Kiani, F. & Moradian, M. ZnS nanoparticles as an efficient and reusable heterogeneous catalyst for synthesis of 1-substituted-1H-tetrazoles under solvent-free conditions. J Nanopart Res 16, 2590 (2014). https://doi.org/10.1007/s11051-014-2590-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2590-0