Abstract
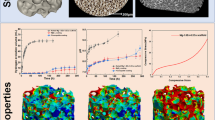
Bioceramics plays an important role in bone-substitutes. In this study, titania porous ceramics with excellent bioactivity were prepared using hydroxyapatite (HA, 10 vol.% contents) as a grain growth inhibitor. The pure TiO2 porous ceramics were also prepared as a control. After sintered at 1,000 °C with a pressureless sintering method, the particle size of the pure TiO2 and TiO2/HA (10 vol.%) porous ceramics were 450 and 310 nm, respectively. Each of the porous ceramics presented numerous pores, which were cross-connected. The size of the pores ranged from 100 to 300 μm. There were also profuse micropores inside the pore wall and between the particles. A SBF soaking experiment demonstrated that the HA additive played an important role in promoting apatite formation. The cell proliferation demonstrated that osteoblasts on the TiO2/HA (10 vol.%) porous ceramics proliferated faster than that on the pure TiO2 ceramics. The histological sections and EDX assay results of the two porous ceramics also illustrated that TiO2/HA (10 vol.%) composite ceramics combined with Ca and P elements induced much better apatite formation than that of the pure TiO2 ceramics. These results indicated that titania ceramics combined with HA holds great promise for bone-substitutes.










Similar content being viewed by others

References
Aksakal B, Yildirim S, Gul H (2007) Metallurgical failure analysis of various implant materials used in orthopedic applications. J Fail Anal Prev 4:17–23
Dimitrievska S, Bureau MN, Antoniou J, Mwale F, Petit A, Lima RS, Marple BR (2011) Titania-hydroxyapatite nanocomposite coatings support human mesenchymal stem cells osteogenic differentiation. J Biomed Mater Res A 98A:576–588
Gordin DM, Gloriant T, Texier G, Thibon I, Ansel D, Duval JL, Nagel MD (2005) Synthesis, structure and electrochemical behavior of a beta Ti–12Mo–5Ta alloy as new biomaterial. Mater Lett 59:2936–2941
Jensen H, Soloview A, Sogaard EG (2005) XPS and FTIR investigation of the surface properties of different prepared titania nano-powders. Appl Surf Sci 246:239–249
Kawashita M, Kim HM, Kokubo T (2003) Apatite-forming ability of carboxyl group-containing polymer gels in a simulated body fluid. Biomaterials 24:2477–2484
Lee SH, Kim HW, Lee EJ (2006) Hydroxyapatite TiO2 hybrid coating on Ti implants. J Biomater Appl 20:195–208
Leonor IB, Baran ET, Kawashita M (2008) Growth of a bonelike apatite on chitosan microparticles after a calcium silicate treatment. Acta Biomater 4:1349–1359
Liu HN, Webster TJ (2011) Enhanced biological and mechanical properties of well-dispersed nanophase ceramics in polymer composites: from 2D to 3D printed structures. Mater Sci Eng C 31:77–89
Michiko S, Elliott B, Webster TJ (2009) Enhanced osteoblast adhesion on hydrothermally treated hydroxyapatite/titania/poly(lactide-co-glycolide) sol–gel titanium coatings. Biomaterals 20:6143–6150
Piveteau LD, Moner I, Girona M (1999) Thin films of calcium phosphate and titanium dioxide by a sol–gel route: a new method for coating medical implants. J Mater Sci Mater Med 10:161–167
Schwartz AD, Mardinger O, Levin L (2009) Marginal bone loss pattern around hydroxyapatite-coated versus commercially pure titanium implants after up to 12 years of follow-up. Int J Oral Maxillofac Implants 20:238–244
Seramak T, Serbinski W (2010) Preparation of the porous biomaterial based on titanium alloy for orthopaedic implants. J Biomech 43:S55–S56
Webster TJ, Ejiofor JU (2004) Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 25:4731–4739
Xu LC, Siedlecki CA (2007) Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 28:3273–3283
Yang BC, Uchida M, Kim HM (2004) Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 25:1003–1010
Yazici H, Ergun C, Bermek H (2008) An in vitro evaluation of the Ca/P ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta Biomater 4:1472–1479
Zhou Y, Niinomi M, Akahori T (2005) Corrosion resistance and biocompatibility of Ti–Ta alloys for biomedical application. Mater Sci Eng A 398:28–36
Acknowledgments
This work was supported by the National Basic Research Program of China (2011CB964701), National Natural Science Foundation of China (81000670), and the key scientific and technological projects of Chongqing (2011AC5025).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Yang, X., Guo, H. et al. Hydroxyapatite additive influenced the bioactivity of bioactive nano-titania ceramics and new bone-forming capacity. J Nanopart Res 14, 1145 (2012). https://doi.org/10.1007/s11051-012-1145-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1145-5