Abstract
In the present study, solid-solution gold–platinum (Au–Pt) nanoparticles with controllable compositions were fabricated by high-intensity femtosecond laser irradiation of an aqueous solution of gold and platinum ions without any chemicals and complicated processes. Transmittance electron microscopy revealed that the single nanometer-sized particles were fabricated by femtosecond laser irradiation of mixed aqueous solutions of gold and platinum ions. The crystalline structure of nanoparticles was characterized by electron and X-ray diffractions. Contrary to the bulk Au–Pt binary systems, which commonly contain a pair of diffraction peaks between pure gold and platinum peaks because of its large miscibility gap in phase diagram, or mixture of Au and Pt, the diffraction peaks of Au–Pt nanoparticles fabricated in the experiment showed a characteristic of the fcc-type lattice. Moreover, the diffraction patterns shifted monotonically from the peak position of pure gold to that of pure platinum as the fractions of platinum ions in the solution were increased. These observations strongly imply that the Au–Pt nanoparticles were solid solution with intended compositions. This technique is not only simple and environmentally friendly, but also applicable to other binary and ternary systems.
Similar content being viewed by others
Introduction
Binary alloy nanoparticles have been intensively studied, especially in the field of catalysis (Reddington et al. 1998; Liu and Smotkin 2002), because they exhibit bifunctional catalytic properties. Gold–platinum (Au–Pt) alloy nanoparticles are currently attracting considerable interests as electrocatalysts for fuel cells (Lou et al. 2001; Stamenkovic et al. 2007). Au–Pt alloy nanoparticles are expected to exhibit synergistic catalytic effects such as suppressing the adsorption of poisonous species (e.g., carbon monoxide on Pt atoms) and modifying the electronic band structure to alter the strength of the surface adsorption. Reduction of the activation energy, which promotes oxidative desorption and suppresses carbon monoxide adsorption, is considered to give rise to an adsorptivity that is sufficiently high to support catalytic oxidation in alkaline electrolytes (Morita et al. 1991; Burke et al. 2000).
Solid-solution Au–Pt alloys have not been generally produced in bulk, because Au–Pt bulk alloy has a large miscibility gap in its phase diagram. This gap arises due to the different electronic energy levels of gold and platinum. However, single nanometer-sized particles strongly tend to form homogeneous alloys because all the atoms retain their electronic structures so that no rehybridization occurs due to band formation (Bond 2007). Au–Pt alloy nanoparticles have been generally prepared by chemical processes (Chen et al. 2006; Mott et al. 2007; Fernández et al. 2007; Lee et al. 2008) on special substrates such as SiO2 (Schrinner et al. 2008) and carbon (Luo et al. 2005, 2006). However, the chemical method does not result in the formation of solid-solution Au–Pt alloy nanoparticles because of the large miscibility gap and the different reduction potentials of Au and Pt. Accordingly, the synthesis commonly involves complex procedures and often employs highly reactive chemicals such as hydrazine (H2NNH2) that may cause environmental and biological problems. Furthermore, the addition of a dispersant is sometimes not suitable for practical applications.
We have recently demonstrated a method for preparing metal nanoparticles of gold (Nakamura et al. 2008), platinum (Nakamura et al. 2009), and silver (Nakamura et al. 2011) that just involves high-intensity laser irradiation of a metal ion solution. The formation of metal nanoparticles by the intense laser irradiation of a solution of metallic ions can be attributed to the generation of highly energetic solvated electrons and hydrogen radicals produced by the laser irradiation. Using this technique, it is expected to produce various pure and alloy metal nanoparticles directly in the solution without any complex procedures or harmful chemicals. In this study, we prepared solid-solution Au–Pt alloy nanoparticles by high-intensity laser irradiation of a mixed solution of gold and platinum ions. The effects of the gold and platinum ion fractions in the solution on the compositions and structures of the Au–Pt alloy nanoparticles were investigated.
Experimental
Sample preparation
The mixed solutions with different gold and platinum molar ratios were prepared by the following procedure. Gold and platinum aqueous solutions were separately prepared by dissolving hydrogen tetrachloroauric (III) tetrahydrate powder (HAuCl4·4H2O, Wako Pure Chemical Industries, Ltd., >99.9%) and hydrogen hexachloroplatinate(IV) hexahydrate powder (H2PtCl6·6H2O, Sigma-Aldrich Co., >99.9%) in ultrapure water. Both solutions had a concentration of 5 × 10−4 M, and they were mixed with different molar ratios. Samples were denoted by the molar fraction of gold and platinum ions in the solution. For example, Au100 and Pt100 denote the solutions containing 100% of gold ions and platinum ions, respectively. Similarly, Au50 denotes the solution containing 50% of gold ions (and 50% platinum ions). All the solutions were transparent and no apparent difference between them was observed. Figure 1a shows UV–Vis absorption spectra of the solutions prepared with different molar fractions of gold and platinum ions using an UV–Vis spectrophotometer (Jasco Co., V630 iRM). As the molar fraction of gold ions decreases, the absorption spectra of the mixed solution vary continuously from that of a pure gold solution (Au100) to that of a pure platinum solution (Pt100).
Laser irradiation condition
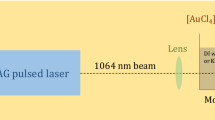
As a target of laser irradiation, 3 mL of each aqueous solution was dispensed in a 10 × 10 × 45 mm quartz glass cuvette that is optically transparent at the incident laser wavelength. Femtosecond laser pulses (wavelength: 800 nm; pulse energy: 5 mJ; pulse width: 100 fs; repetition rate: 30 Hz) were generated from a chirped-pulse amplified Ti:sapphire laser. The laser beam was introduced on the cuvette and tightly focused in the solution by an aspheric lens (focal length: 8 mm; numerical aperture: 0.5). The spot diameter was estimated to be 175 μm by taking account of the spherical aberration due to the refractive index mismatching. Thus, the laser intensity was estimated to be 2.1 × 1014 W/cm2. An irradiation time of 30 min was used in all the experiments. The detailed experimental setup has been shown in the reference (Nakamura et al. 2011).
Characterization
The optical characteristics of the solutions after laser irradiation were evaluated using the UV–Vis spectrophotometer. Transmission electron microscopy (TEM; JEOL, JEM2000EXII) micrographs of the products were obtained after irradiation. The TEM samples were prepared by dropping a few drops of the solution on a carbon-coated copper grid (Okenshoji Co., Ltd., microgrid B) immediately after the irradiation and drying in air at room temperature. X-ray diffraction (XRD, RINT-V, Rigaku Co. with Cu Kα irradiation, λ = 0.154056 nm) was used to analyze the crystalline structure of fabricated particles. The samples for XRD were prepared by placing freeze-dried powder on a non-reflecting single-crystal silicon plate (Rigaku Co.), which contained no diffraction peaks over the measurement angle range.
Results and discussion
Tiny luminescent flash and fine bubbles were simultaneously observed near the focal point during laser irradiation. These bubbles were identified as oxygen and hydrogen by a chromatographic analysis (Shimadzu Co., GC-8A). These bubbles were probably produced by the decomposition of water molecules due to laser-induced breakdown (Chin and Lagacé 1996). The solutions were initially transparent but gradually became colored during the laser irradiation. The final color of the solutions after 30 min irradiation strongly depended on the molar ratios of gold and platinum ions in the solution: red-purple for Au100 to light-brown for Pt100 as shown in Fig. 2.
Figure 1b shows a representative set of UV–Vis absorption spectra of the solutions with different molar ratios, obtained immediately after irradiation. The spectrum of the gold solution (Au100) contains an absorption peak at 520 nm originated from the surface plasmon resonance of gold nanoparticles. The peak position shifted to shorter wavelength and the absorbance decreased as the molar fraction of gold ions decreased. This represents that the gold nanoparticles were fabricated in the Au100 solution. The formation of alloy nanoparticles will be discussed based on the experimental results of electron and X-ray diffractions.
Figure 3 shows bright-field TEM images of the particles fabricated in the solutions with different molar ratios, and the corresponding particle size distribution is presented in Fig. 4. The mean particle size (d), the standard deviation (SD), and coefficient of variation (CV) of the particles estimated from the TEM images were summarized in Table 1. Single nanometer-sized spherical particles were fabricated in all solutions even though the size distributions were relatively broad (CV = ~60%). Despite the absence of dispersants in the solution, the fabricated particles did not form aggregates and showed relatively good dispersion state just after irradiation. This indicates that these nanoparticles can be supported on the surface of many kinds of matrices without any capping agents, leading to maintain high reactivity of the nanoparticles in practical applications. Moreover, the dispersion state of the nanoparticles lasted several days in the solutions after irradiation. This might be caused by the electrostatic repulsion of the particles formed in the laser irradiation reaction as reported by Sylvestre’s group (Sylvestre et al. 2004). It should be noticed that the smaller mean particle size and the narrower particle size distribution were achieved in the mixed solution compared with those in the gold (Au100) and platinum (Pt100) solutions. The selected area electron diffraction (SAED) profiles using a 300-nm sized aperture were employed to analyze crystalline structure of the particles. The interplanar spacing (d hkl ) of a given lattice plane (hkl) was estimated from the corresponding radius, r, in the SAED patterns using the expression:
where L is the camera length of the instrument (in this setup, L = 82.40 cm) and λ is the wavelength of the electron beam (λ = 0.002507 nm). The SAED pattern of the nanoparticles obtained in Au100 solution is shown in Fig. 5 as a representative example, and the estimated interplanar spacings of nanoparticles fabricated in the mixed solutions and the corresponding lattice planes (hkl) are summarized in Table 2. The interplanar spacings of the fabricated nanoparticles in the Au100 and Pt100 solutions showed in good agreement with the data for (111), (200), (220), and (300) planes of gold and platinum from Joint Committee for Powder Diffraction Standards (JCPDS) card (No. 04-0784 for gold and No. 04-0802 for platinum). On the other hand, the interplanar spacing of the particles fabricated in the mixed solution of gold and platinum ions was changed with increasing the molar fraction of platinum ions in the solutions. Lattice parameter was estimated from the interplanar spacings of fcc structure using the expression:
where a is the lattice parameter and (hkl) is the given lattice plane. Lattice parameters of the nanoparticles estimated by Eq. 2 are plotted in Fig. 7 as a function of platinum molar fraction in the solution. The lattice parameters indicated by closed red circles showed a linear relationship with the platinum molar fraction in the solutions. This follows the Vegard’s law typically observed for binary alloy system, suggesting the alloy formation of the binary Au–Pt nanoparticles.
To determine the structural characteristics of the fabricated particles in more detail, XRD measurements were performed for all the samples. Figure 6 shows a representative set of XRD profiles. The broken lines indicate typical XRD peak positions from (111) and (200) planes of gold and platinum. The diffraction patterns of nanoparticles fabricated in Au100 and Pt100 solutions could be clearly indexed as fcc-type cubic lattice of gold and platinum, respectively. The diffraction patterns of nanoparticles fabricated in the mixed solutions also showed a single peak characteristic of the fcc-type lattice, while two representative peaks of gold and platinum will be observed in the profile of the phase-segregated Au–Pt alloys or physical mixture of gold and platinum (Fernández et al. 2007). Moreover, those diffraction patterns showed a transition from that of gold to platinum with the increase of platinum content in the solution. This strongly indicates that the fabricated nanoparticles possess the characteristics of Au–Pt alloy.
The structural analysis results from the diffraction peaks of (111) and (200) planes for the particles obtained by Integrated X-ray Powder Diffraction Software (Rigaku Co.) are summarized in Table 3. The crystallite sizes of the particles calculated by the Scherrer equation seem to be larger than the particle sizes in the TEM images (Table 1), especially in the gold sample (Au100). This is in a good agreement with the facts that gold particles tend to grow and crystallize faster than other noble metals such as palladium and platinum because of its low melting point (1336 K) and have less affinity to oxygen. Moreover, the fabricated nanoparticles have high-surface activities without any dispersants. This might be caused by the crystal growth inhibition during freeze-drying due to the presence of platinum element in the fabricated particles. Namely, the crystallite sizes decreased from 50 to 6 nm with the decrease in the fraction of gold ions in the solution.
The averaged lattice parameters obtained from two diffraction peaks of (111) and (200) planes of the particles fabricated in the solutions of Au100 (a = 4.081 Å) and Pt100 (a = 3.928 Å) were in a good agreement with those of bulk gold and platinum. Interestingly, the lattice parameters of the fabricated nanoparticles varied almost linearly from bulk gold to bulk platinum with the decrease in the fraction of gold ions in the solutions. The lattice parameters, which were estimated by Eq. 2, also showed a linear relationship with platinum molar fraction in the solution, as shown in Fig. 7 (open and closed blue squares). This clearly demonstrates that solid-solution Au–Pt nanoparticles with the intended compositions were fabricated in the solutions by high-intensity laser irradiation of solutions without any reduction agents.
The formation mechanism of Au–Pt nanoparticles by laser irradiation of the solution without any reducing agents is attributed to the optically induced decomposition of water molecules. Namely, solvated electrons and hydrogen radicals are formed in the aqueous solutions by a photochemical reaction (Huang et al. 1996; Henglein 1998; Kempa et al. 2006). The generation of oxygen and hydrogen near the focal spot during laser irradiation (Chin and Lagacé 1996) strongly indicates that hydrogen and hydroxyl radicals are simultaneously produced in the solution. Solvated electrons and hydrogen radicals have a role as strong reducing agents in the solution. Consequently, metal ions in the solution are readily reduced to zero-valence atoms. When the particles become several nanometers in size, most of the atoms produced by the laser irradiation have been expended and the particles ceased to grow.
The sizes of the nanoparticles in the present study were of the order of nanometers and their compositions exhibited a relatively good agreement with the molar fraction of the solution. This is due to the strong reducing power of solvated electrons and hydrogen radicals produced by high-intensity laser irradiation of the aqueous solutions. By this mechanism, solid-solution Au–Pt nanoparticles were fabricated by high-intensity laser irradiation of aqueous solution without any reducing agents.
Conclusion
In summary, we have fabricated solid-solution Au–Pt nanoparticles with controllable compositions from the mixed solution of gold and platinum ions in a high-intensity optical field produced by tightly focused femtosecond laser pulses. This technique is simple and environmentally friendly and does not use any chemicals except for metallic salts. Furthermore, it can be applied to synthesize other binary and ternary systems.
References
Bond GC (2007) The electronic structure of platinum–gold alloy particles. Platinum Met Rev 51:63–68. doi:10.1595/147106707X187353
Burke LD, Collins JA, Horgan MA, Hurley LM, O’Mullane AP (2000) The importance of the active states of surface atoms with regard to the electrocatalytic behaviour of metal electrodes in aqueous media. Electrochim Acta 45:4127–4134. doi:10.1016/S0013-4686(00)00532-6
Chen HM, Peng HC, Liu RS, Hu SF, Sheu HS (2006) Morphology and surface plasma changes of Au–Pt bimetallic nanoparticles. J Nanosci Nanotechnol 6:1411–1415. doi:10.1166/jnn.2006.199
Chin SL, Lagacé S (1996) Generation of H2, O2, and H2O2 from water by the use of intense femtosecond laser pulses and the possibility of laser sterilization. Appl Opt 35:907–911. doi:10.1364/AO.35.000907
Fernández PH, Rojas S, Ocón P, Gómez de la Fuente JL, Fabián JS, Sanza J, Peña MA, García FJG, Terreros P, Fierro JLG (2007) Influence of the preparation route of bimetallic Pt–Au nanoparticle electrocatalysts for the oxygen reduction reaction. J Phys Chem C 111:2913–2923. doi:10.1021/jp066812k
Henglein A (1998) Colloidal silver nanoparticles: photochemical preparation and interaction with O2, CCl4, and some metal ions. Chem Mater 10:444–450. doi:10.1021/cm970613j
Huang HH, Ni XP, Loy GL, Chew CH, Tan KL, Loh FC, Deng JF, Xu GQ (1996) Photochemical formation of silver nanoparticles in poly(N-vinylpyrrolidone). Langmuir 12:909–912. doi:10.1021/la950435d
Kempa T, Farrer RA, Giersig M, Fourkas JT (2006) Photochemical synthesis and multiphoton luminescence of monodisperse silver nanocrystals. Plasmonics 1:45–51. doi:10.1007/s11468-006-9008-5
Lee JK, Lee J, Hanc J, Limc TH, Sungd YE, Tak Y (2008) Influence of Au contents of AuPt anode catalyst on the performance of direct formic acid fuel cell. Electrochim Acta 53:3474–3478. doi:10.1016/j.electacta.2007.12.031
Liu RX, Smotkin ES (2002) Array membrane electrode assemblies for high throughput screening of direct methanol fuel cell anode catalysts. J Electroanal Chem 535:49–55. doi:10.1016/S0022-0728(02)01144-0
Lou Y, Maye MM, Han L, Luo J, Zhong CJ (2001) Gold–platinum alloy nanoparticle assembly as catalyst for methanol electrooxidation. Chem Commun 5:473–474. doi:10.1039/b008669j
Luo J, Maye MM, Petkov V, Kariuki NN, Wang L, Njoki P, Mott D, Lin Y, Zhong CJ (2005) Phase properties of carbon-supported gold–platinum nanoparticles with different bimetallic compositions. Chem Mater 17:3086–3091. doi:10.1021/cm050052t
Luo J, Njoki PN, Lin Y, Mott D, Wang L, Zhong CJ (2006) Characterization of carbon-supported AuPt nanoparticles for electrocatalytic methanol oxidation Reaction. Langmuir 22:2892–2898. doi:10.1021/la0529557
Morita M, Iwanaga Y, Matsuda Y (1991) Anodic oxidation of methanol at a gold-modified platinum electrocatalyst prepared by RF sputtering on a glassy carbon support. Electrochim Acta 36:947–951. doi:10.1016/0013-4686(91)85299-M
Mott D, Luo J, Njoki PN, Lin Y, Wang L, Zhong CJ (2007) Synergistic activity of gold–platinum alloy nanoparticle catalysts. Catal Today 122:378–385. doi:10.1016/j.cattod.2007.01.007
Nakamura T, Mochidzuki Y, Sato S (2008) Fabrication of gold nanoparticles in intense optical field by femtosecond laser irradiation of aqueous solution. J Mater Res 23:968–974. doi:10.1557/JMR.2008.0115
Nakamura T, Takasaki K, Ito A, Sato S (2009) Fabrication of platinum particles by intense, femtosecond laser pulse irradiation of aqueous solution. Appl Surf Sci 255:9630–9633. doi:10.1016/j.apsusc.2009.04.092
Nakamura T, Magara H, Herbani Y, Sato S (2011) Fabrication of silver nanoparticles by highly intense laser irradiation of aqueous solution. Appl Phys A 104:1021–1024. doi:10.1007/s00339-011-6499-5
Reddington E, Sapienza A, Gurau B, Viswanathan R, Sarangapani S, Smotkin ES, Mallouk TE (1998) Combinatorial electrochemistry: a highly parallel, optical screening method for discovery of better electrocatalysts. Science 280:1735–1737. doi:10.1126/science.280.5370.1735
Schrinner M, Proch S, Mei Y, Kempe R, Miyajima N, Ballauff M (2008) Stable bimetallic gold–platinum nanoparticles immobilized on spherical polyelectrolyte brushes: synthesis, characterization, and application for the oxidation of alcohols. Adv Mater 20:1928–1933. doi:10.1002/adma.200702421
Stamenkovic VR, Mun BS, Arenz M, Mayrhofer KJJ, Lucas CA, Wang G, Ross PN, Markovic NM (2007) Trends in electrocatalysis on extended and nanoscale Pt–bimetallic alloy surfaces. Nat Mater 6:241–247. doi:10.1038/nmat1840
Sylvestre JP, Poulin S, Kabashin AV, Sacher E, Meunier M, Luong JHT (2004) Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J Phys Chem B 108:16864–16869. doi:10.1021/jp047134+
Acknowledgments
This study was financially supported by a Grant-in-Aid for Young Scientists (B) (No. 21760575) from the Japan Society for the Promotion of Science and the Adaptable and Seamless Technology Transfer Program through target-driven R&D from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, T., Herbani, Y. & Sato, S. Fabrication of solid-solution gold–platinum nanoparticles with controllable compositions by high-intensity laser irradiation of solution. J Nanopart Res 14, 785 (2012). https://doi.org/10.1007/s11051-012-0785-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0785-9