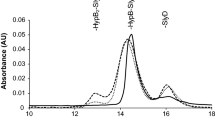
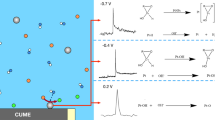
Abstract
A dimeric hydrogenase enzyme (44.5 and 39.4 kDa sub units) was isolated in a 39.5% yield from the fungus Fusarium oxysporum and purified 4.64-fold by ion exchange chromatography on Sephacryl S-200. Characterisation of the enzyme afforded pH and temperature optima of 7.5 and 38 °C, respectively, a half-life stability of 36 min and a V max and K m of 3.57 nmol min−1 mL−1 and 2.25 mM, respectively. This enzyme was inhibited (non-competitively) by hydrogen hexachloroplatinic acid (H2PtCl6) at 1 or 2 mM with a K i value of 118 μM. Incubation of the platinum salt with the pure enzyme under an atmosphere of hydrogen and optimum enzyme conditions (pH 7.5, 38 °C) afforded <10% bioreduction after 8 h while at conditions suitable for platinum nanoparticle formation (pH 9, 65 °C) over 90% reduction took place after the same length of time. Cell-free extract from the fungal isolates produced nearly 90% bioreduction of the platinum salt under both pH and temperature conditions. The bioreduction of the platinum salt by a hydrogenase enzyme takes place by a passive process and not an active one as previously understood.







Similar content being viewed by others
References
Ahmad A, Mukherjee P, Mandal D, Senapati S, Khan MI, Kumar R et al (2002) Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus Fusarium oxysporum. J Am Chem Soc 124:12108–12109. doi:10.1021/ja027296o
Ahmad A, Senepati S, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 19:3550–3553. doi:10.1021/la026772l
Ahmad A, Senepati S, Khan MI, Kumar R, Sastry M (2005) Extra-/Intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Tricothecium sp. J Biomed Nanotechnol 1:47–53. doi:10.1166/jbn.2005.012
Bhainsa KC, D’Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces 47:160–164. doi:10.1016/j.colsurfb.2005.11.026
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Brust M, Kiely CJ (2002) Some recent advances in nanostructure preparation from gold and silver particles: a short topical review. J Coll Surf A 202:175–186. doi:10.1016/S0927-7757(01)01087-1
De Luca G, de Philip P, Dermoun Z, Rousset M, Vermeglio A (2001) Reduction of technetium (VII) by Desulfovibrio fructosovorans is mediated by the nickel–iron hydrogenase. Appl Environ Microbiol 67:4583–4587. doi:10.1128/AEM.67.10.4583-4587.2001
Duff DG, Edwards PP, Johnson BFG (1995) Formation of a polymer-protected platinum sol: a new understanding of the parameters controlling morphology. J Phys Chem 99:15934–15944. doi:10.1021/j100043a036
Elliot SJ, Leger C, Pershad HR, Hirst J, Heffron K, Ginet N et al (2002) Detection and interpretation of redox potential optima in the catalytic activity of enzymes. Biochim Biophys Acta 1555:54–59. doi:10.1016/S0005-2728(02)00254-2
Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troianl HE, Santiago P et al (2002) Formation and growth of Au nanoparticles inside alfalfa plants. Nanotechnol Lett 2:397–401
He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate. Mater Lett 61:3984–3987. doi:10.1016/j.matlet.2007.01.018
Huang H, Yang X (2005) One-step, shape control synthesis of gold nanoparticles stabilized by 3-thiopheneacetic acid. Colloids Surf A Physicochem Eng Asp 255:11–17. doi:10.1016/j.colsurfa.2004.12.020
Huang J, He C, Liu X, Xiao Y, Mya KY, Chai J (2004) Formation and characterisation of water soluble platinum nanoparticles using a unique approach based on the hydrosilylation reaction. Langmuir 20:5145–5148. doi:10.1021/la036135a
Huang J, Liu Z, Liu X, He C, Chow SY, Pan J (2005) Platinum nanoparticles from the hydrosilylation reaction: capping agents, physical characterisations, and electrochemical properties. Langmuir 21:699–704. doi:10.1021/la0482148
Kamachi T, Uno S, Hiraoshi T, Okura I (1995) Purification and properties of intact hydrogenase from Desulfovibrio vulgaris (Miyazaki). J Mol Catal Chem 95:93–98. doi:10.1016/1381-1169(94)00147-2
Klaus T, Joerger R, Olsson E, Granqviat GC (1999) Silver based crystalline nanoparticles, microbial fabricated. Proc Natl Acad Sci USA 96:13611–13614. doi:10.1073/pnas.96.24.13611
Klaus-Joerger T, Joerger R, Olsson E, Granqvist CG (2001) Bacteria as workers in the living factory: metal accumulating bacteria and their potential for materials science. Trends Biochem Sci 19:15–20
Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H et al (2007) Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 128:648–653. doi:10.1016/j.jbiotec.2006.11.014
Kowshik M, Ashtaputre S, Kharrazi T, Vogel W, Urban J, Kulkarni SK et al (2003) Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14:95–100. doi:10.1088/0957-4484/14/1/321
Kumar A, Joshi HM, Mandal AB, Srivastava R, Adyanthaya SD, Pasricha R et al (2004) Phase transfer of platinum nanoparticles from aqueous to organic solutions using fatty amine molecules. J Chem Sci 116:293–300. doi:10.1007/BF02708280
Lengke MF, Fleet ME, Southam G (2006) Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum (IV)—chloride complex. Langmuir 22:7318–7323. doi:10.1021/la060873s
Liu Z, Ling XY, Su X, Lee JY (2004) Carbon supported Pt and PtRu Nanoparticles as catalysts for a direct methanol fuel cell. J Phys Chem B 108:8234–8240. doi:10.1021/jp049422b
Liu Z, Shamsuzzoha M, Ada ET, Reichat M, Nikles DE (2007) Synthesis and activation of platinum nanoparticles with controlled size for fuel cell electrocatalysts. J Power Sour 164:472–480. doi:10.1016/j.jpowsour.2006.10.104
Lloyd JR (2003) Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425. doi:10.1016/S0168-6445(03)00044-5
Lloyd JR, Sole VA, Van Praagh CV, Lovely DR (2000) Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl Environ Microbiol 66:3743–3749. doi:10.1128/AEM.66.9.3743-3749.2000
Lovely DR, Widman PK, Woodward JC, Phillips EJ (1993) Reduction of uranium by cytochrome c3 of Desulfovibrio vulgaris. Appl Environ Microbiol 59:3572–3576
Mukherjee P, Senepati S, Mandal D, Ahmad A, Khan MI, Kumar R et al (2002) Extracellular synthesis of gold nanoparticles by the fungus, Fusarium oxysporum. Chembiochem 5:461–463. doi :10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X
Nadagouda MN, Varma RS (2006) Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: density assisted self assembly of nanospheres, wires and rods. Green Chem 8:516–518. doi:10.1039/b601271j
Nair B, Pradeep T (2002) Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des 2:293–298. doi:10.1021/cg0255164
Ngwenya N, Whiteley CG (2006) Recovery of rhodium (III) from solutions and industrial wastewaters by a sulphate reducing consortium. Biotechnol Prog 22:1604–1611. doi:10.1021/bp060167h
Peng ZA, Peng X (2001) Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as a precursor. J Am Chem Soc 123:183–184. doi:10.1021/ja003633m
Qu L, Peng ZA, Peng X (2001) Alternative routes toward high quality CdSe nanocrystals. Nano Lett 1:333–337. doi:10.1021/nl0155532
Rashamuse K, Whiteley CG (2007) Bioreduction of platinum (IV) from aqueous solution using sulphate reducing bacteria. Appl Microbiol Biotechnol 75:1429–1435. doi:10.1007/s00253-007-0963-3
Riddin TL, Gericke M, Whiteley CG (2006) Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f.sp. Lycopersici using response surface methodology. Nanotechnology 17:1–8. doi:10.1088/0957-4484/17/1/001
Shahverdi AR, Miniaeian S, Shahverdi HR, Jamalifar H, Nohi AA (2007) Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem 42:919–923. doi:10.1016/j.procbio.2007.02.005
Shukla N, Svedberg EB, Ell J (2007) Surfactant isomerization and dehydrogenation of FePt nanoparticles. Colloids Surf A Physicochem Eng Asp 301:113–116
Tang Z, Geng D, Lu G (2005) Size controlled synthesis of colloid platinum nanoparticles and their catalytic activity for the electrocatalytic oxidation of carbon monoxide. J Coll Inter Sci 287:159–166. doi:10.1016/j.jcis.2005.01.096
Willner I, Baron R, Willner B (2006) Growing metal nanoparticles by enzymes. Adv Math 18:1109–1120. doi:10.1002/adma.200501865
Winter G, Buhrke T, Lenz O, Jones AK, Forgber M, Friedrich B (2005) A model system for [NiFe] hydrogenase maturation studies: purification of an active site containing hydrogenase large subunit without small subunit. FEBS Lett 579:4292–4296. doi:10.1016/j.febslet.2005.06.064
Xiao Y, Pavlov V, Levine S, Niazov T, Markovitch G, Willner I (2004) Catalytic growth of Au nanoparticles by NAD (P) H cofactors: optical sensors for NAD (P)+-dependent biocatalyzed transformations. Angew Chem Int Ed Engl 43:4519–4522. doi:10.1002/anie.200460608
Yang W, Ma Y, Tang J, Yang X (2007) Green synthesis of monodisperse platinum nanoparticles and their catalytic properties. Colloids Surf A Physicochem Eng Asp 302:628–633. doi:10.1016/j.colsurfa.2007.02.028
Acknowledgements
Financial assistance for YG and TR from MINTEK (South Africa) is gratefully appreciated
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Govender, Y., Riddin, T.L., Gericke, M. et al. On the enzymatic formation of platinum nanoparticles. J Nanopart Res 12, 261–271 (2010). https://doi.org/10.1007/s11051-009-9604-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-009-9604-3