Abstract
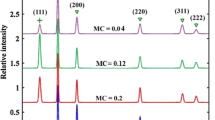
The optimal parameters are found for preparing nanofluid in our submerged arc nanoparticle synthesis system (SANSS) using a copper electrode. A suspended copper oxide nanofluid is thus produced at the current of 8.5–10 A, voltage of 220 V, pulse duration of 12 μs, and dielectric liquid temperature of 2°C. The CuO nanoparticle are characterized by transmission electron microscopy (TEM), field emission scanning electron microscope (FESEM), X-ray diffraction (XRD), electron diffraction pattern (SAD) and electron spectroscopy for chemical analysis (ESCA). The equality volume spherical diameter of the obtained copper oxide particle is 49.1 nm, regular shape and narrow size distribution.
Similar content being viewed by others
References
K. Borgohain J.B. Singh M.V. Rama Rao T. Shripathi S. Mahamuni (2000) ArticleTitleQuantum size effects in CuO nanoparticles Phys. Rev. B 61 11093–11096 Occurrence Handle10.1103/PhysRevB.61.11093
H. Chang T.T. Tshih C.-R. Lin H.M. Lin C.K. Lin C.H. Lo H.T. Su (2004) ArticleTitleA study of magnetic field effect on nanofluid stability of CuO Mater. Trans. 45 IssueID4 1375–1378
P.C. Dai H.A. Mook G. Aeppli S.M. Hayden F. Dogan (2000) ArticleTitleResonance as a measure of pairing correlations in the high-Tc superconductor YBa2Cu3O6.6 Nature 406 965–968 Occurrence Handle10.1038/35023094 Occurrence Handle10984044
J.F. Deng Q. Sun Y.L. Zhang S.Y. Chen D. Wu (1996) ArticleTitleA novel process for preparation comparison of various preparation methods of a Cu/ZnO/Al2O3 ultrafine catalyst for methanol synthesis from CO2+H2: comparison of various preparation methods. Appl. Catal. A 139 75–85 Occurrence Handle10.1016/0926-860X(95)00324-X
A.A. Eliseev A.V. Lukashin A.A. Vertegel L.I. Heifets A.I. Zhirov Y.D. Tretyakov (2000) ArticleTitleMater Res. Innovations 3 308–315 Occurrence Handle10.1007/s100190000052
P.T. Eubank M.R. Patel M.A. Barrufet B. Bozurt (1993) ArticleTitleTheoretical models of the electrical discharge machining process. III The variable mass, cylindrical plasma model. J. Appl. Phys. 73 IssueID11 7900–7909
M. Frietsch F. Zudock J. Goschnick M. Bruns (2000) ArticleTitleCuO catalytic membrane as selectivity trimmer for metal oxide gas sensors Sens. Actuat B 65 379–381 Occurrence Handle10.1016/S0925-4005(99)00353-6
R.V. Kumar Y. Diamant A. Gedanken (2000) ArticleTitleSonochemical synthesis and characterization of nanometer-size transition metal oxides from metal acetates Chem. Mater. 12 2301–2305 Occurrence Handle10.1021/cm000166z
T. Maruyama (1998) ArticleTitleCoppy oxide thin films prepared by chemical vapor deposition from copper dipivaloylmethanate Sol. Energy Mater. Sol. Cells 56 85–92
S. Nakao M. Ikeyama T. Mizota P. Jin M. Tazawa Y. Miyagawa S. Miyagawa S. Wang L. Wang (2000) ArticleTitleRep. Res Cent. Ion Beam Technol., Hosei Univ. Suppl. 18 153–160
M.R. Patel T. MariaA.Barrufet Philip D. Eubank Daryl DiBitonto. (1989) ArticleTitleTheoretical models of the electrical discharge machining process. II. The anode erosion model J. Appl. Phys. 66 IssueID9 4104–4111 Occurrence Handle10.1063/1.343995
T.T. Tshih H. Chang L.C. Chen L.L. Han C.H. Lo M.K. Liu (2003) ArticleTitleDevelopment of pressure control technique of an arc-submerged nanoparticle synthesis system (ASNSS) for copper nanoparticle fabrication Mater. Trans. 44 IssueID6 1138–1142
C. Xu Y. Liu G. Xu G. Wang (2002) ArticleTitlePreparation and characterization of CuO nanorods by thermal decomposition of CuC2O4 precursor Mater. Res. Bull. 37 2365–2372 Occurrence Handle10.1016/S0025-5408(02)00848-6
J.F. Xu W. Ji Z.X. Shen S.H. Tang X.R. Ye D.Z. Jia X.Q. Xin (2000) ArticleTitlePreparation and characterization of CuO nanocrystals J. Solid State Chem. 147 516–519 Occurrence Handle10.1006/jssc.1999.8409
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo, CH., Tsung, TT., Chen, LC. et al. Fabrication of copper oxide nanofluid using submerged arc nanoparticle synthesis system (SANSS). J Nanopart Res 7, 313–320 (2005). https://doi.org/10.1007/s11051-004-7770-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11051-004-7770-x