Abstract
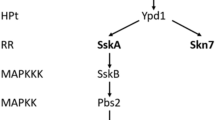
Aspergillus fumigatus is naturally exposed to a highly variable environment and subjected to various kinds of stresses. High-osmolarity glycerol mitogen-activated protein kinase (HOG-MAPK) pathway plays a crucial role in regulating cellular homeostasis in response to environmental changes. Here, we explored the contribution of HOG-MAPK pathway to the adaptive responses to thermal stress and other related stresses in A. fumigatus. We observed the phenotype features of wild-type strains and their derived mutants at 37 and 48 °C, and the results suggested that tcsB participates in response to high temperature. Furthermore, susceptibility test for antifungal drugs showed that SHO1 branch is probably involved in the susceptibility of A. fumigatus to itraconazole at high temperature. Although sakA expression at mRNA level appeared unchanged in wild-type AF293 subjected to thermal stress, phosphorylated SakAp level increased significantly in the strains exposed to cold stress, 250 mmol/L nystatin or 10 % dimethyl sulfoxide in a manner dependent on the SLN1 branch and independent on the SHO1 branch. Taken together, these results indicate that HOG-MAPK pathway, especially the SLN1 branch, plays an important role in the adaptations of A. fumigatus to thermal stress and other related stresses.




Similar content being viewed by others
References
Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–50.
Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803.
Kontoyiannis DP, Bodey GP. Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis. 2002;21:161–72.
Perfect JR. Antifungal resistance: the clinical confront. Oncology. 2004;18:15–22.
Bahn YS. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot Cell. 2008;7:2017–36.
Gustin MC, Albertyn J, Alexander M, et al. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–300.
Kawasaki L, Sánchez O, Shiozaki K, et al. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol Microbiol. 2002;45:1153–63.
Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–9.
Hirt H. Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci. 1997;2:11–5.
Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–97.
Roychoudhury S, Zielinski NA, Ninfa AJ, et al. Inhibitors of two-component signal transduction systems: inhibition of alginate gene activation in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1993;90:965–9.
López-Goñi I, Guzmán-Verri C, Manterola L, et al. Regulation of Brucella virulence by the two-component system BvrR/BvrS. Vet Microbiol. 2002;90:329–39.
Hayashi J, Nishikawa K, Hirano R, et al. Identification of a two-component signal transduction system involved in fimbriation of Porphyromonas gingivalis. Microbiol Immunol. 2000;44:279–82.
Moskowitz SM, Ernst RK, Miller SI, et al. A two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol. 2004;186:575–9.
Brewster JL, de Valoir T, Dwyer ND, et al. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–3.
Lawrence CL, Botting CH, Antrobus R, et al. Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Mol Cell Biol. 2004;24:3307–23.
Winkler A, Arkind C, Mattison CP, et al. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway and protein tyrosine phosphatases are essential under heat stress. Eukaryot Cell. 2002;2:163–73.
Alonso-Monge R, Real E, Wojda I, et al. Hyperosmotic stress response and regulation of cell wall in Saccharomyces cerevisiae share common functional aspects. Mol Microbiol. 2001;41:717–30.
Gacto M, Soto T, Vicente-Soler J, et al. Learning from yeasts: intracellular sensing of stress conditions. Int Microbiol. 2003;6:211–9.
Hayashi M, Maeda T. Activation of the HOG Pathway upon cold stress in Saccharomyces cerevisiae. J Biochem. 2006;139:797–803.
Hospenthal DR, Kwon-Chung KJ, Bennett JE. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol. 1998;36:165–8.
Ma Y, Qiao J, Liu W, et al. The Sho1 sensor regulates growth, morphology, and oxidant adaptation in Aspergillus fumigatus but is not essential for development of invasive pulmonary aspergillosis. Infect Immun. 2008;76:1695–701.
马彦,乔建军,刘伟, 等. 烟曲霉pbs2基因功能初步探讨. 中华微生物学和免疫学杂志. 2008;28:1126–30.
Du C, Sarfati J, Latge JP, et al. The role of the sakA (Hog1) and tcsB (sln1) genes in the oxidant adaptation of Aspergillus fumigatus. Med Mycol. 2006;44:211–8.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard-second edition. CLSI document M38-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
Shiozaki K, Shiozaki M, Russell P. Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–49.
Hartmann T, Sasse C, Schedler A, et al. Shaping the fungal adaptome–stress responses of Aspergillus fumigatus. Med Microbiol. 2011;301:408–16.
Abad A, Fernández-Molina JV, Bikandi J, et al. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol. 2010;27:155–82.
Furukawa K, Katsuno Y, Urao T, et al. Isolation and functional analysis of a gene, tcsB, encoding a transmembrane hybrid-type histidine kinase from Aspergillus nidulans. Appl Environ Microbiol. 2002;68:5304–10.
Alonso-Monge R, Navarro-García F, Román E, et al. The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot Cell. 2003;2:351–61.
Segmüller N, Ellendorf U, Tudzynski B, et al. A stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot Cell. 2007;6:211–21.
Han KH, Prade RA. Osmotic stress-coupled maintenance of polar growth in Aspergillus nidulans. Mol Microbiol. 2002;43:1065–78.
Lenassi M, Vaupotic T, Gunde-Cimerman N, et al. The MAP kinase HwHog1 from the halophilic black yeast Hortea werneckii: coping with stresses in solar salterns. Saline Syst. 2007;3:3–14.
Yang F, Ma D, Wan Z, et al. The role of sho1 in polarized growth of Aspergillus fumigatus. Mycopathologia. 2011;172:347–55.
Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300–72.
Orvar BL, Sangwan V, Omann F, et al. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23:785–94.
Sangwan V, Orvar BL, Beyerly J, et al. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 2002;31:629–38.
Hayashi M, Maeda T. Activation of the HOG pathway upon cold Stress in Saccharomyces cerevisiae. J Biochem. 2006;139:797–803.
Mejía R, Gómez-Eichelmann MC, Fernández MS. Membrane fluidity of Escherichia coli during heat shock. Biochim Biophys Acta. 1995;1239:195–200.
Swan TM, Watson K. Membrane fatty acid composition and membrane fluidity as parameters of stress tolerance in yeast. Can J Microbiol. 1997;43:70–7.
Rodríguez-Peña JM, García R, Nombela C, et al. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: a yeast dialogue between MAPK routes. Yeast. 2010;27:495–502.
Bahn YS, Geunes-Boyer S, Heitman J. Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress-activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryot Cell. 2007;6:2278–89.
Acknowledgments
We thank Prof. J. P. Latgé (Institute Pasteur, Paris, France) for his constructive comments on this study and the gifts of A. fumigatus △sakA, △tcsB, and wild-type DAL strains. This project was supported by the National Fund of Natural Sciences in China (No.30930006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, Y., Yang, F., Ma, D. et al. HOG-MAPK Signaling Regulates the Adaptive Responses of Aspergillus fumigatus to Thermal Stress and Other Related Stress. Mycopathologia 174, 273–282 (2012). https://doi.org/10.1007/s11046-012-9557-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-012-9557-4