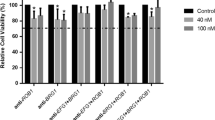
Abstract
The introduction of RNA silencing machinery in fungi has led to the promising application of RNAi methodology to knock down essential vital factor or virulence factor genes in the microorganisms. Efg1p is required for development of a true hyphal growth form which is known to be essential for interactions with human host cells and for the yeast’s pathogenesis. In this paper, we describe the development of a system for presenting and studying the RNAi function on the EFG1 gene in C. albicans. The 19-nucleotide siRNA was designed on the basis of the cDNA sequence of the EFG1 gene in C. albicans and transfection was performed by use of a modified-PEG/LiAc method. To investigate EFG1 gene silencing in siRNA-treated cells, the yeasts were grown in human serum; to induce germ tubes a solid medium was used with the serum. Quantitative changes in expression of the EFG1 gene were analyzed by measuring the cognate EFG1 mRNA level by use of a quantitative real-time RT-PCR assay. Compared with the positive control, true hyphae formation was significantly reduced by siRNA at concentrations of 1 μM, 500 nM, and 100 nM (P < 0.05). In addition, siRNA at a concentration of 1 μM was revealed to inhibit expression of the EFG1 gene effectively (P < 0.05). On the basis of the potential of post-transcriptional gene silencing to control the expression of specific genes, these techniques may be regarded as promising means of drug discovery, with applications in biomedicine and functional genomics analysis.




Similar content being viewed by others
References
Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5(4):366–71.
Rooney PJ, Klein BS. Linking fungal morphogenesis with virulence. Cell Microbiol. 2002;4(3):127–37.
Odds FC. Candida and candidosis: a review and bibliography. London: Baillière Tindall; 1988.
Phan QT, Belanger PH, Filler SG. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun. 2000;68(6):3485–90.
Srikantha T, Tsai LK, Daniels K, Soll DR. EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J Bacteriol. 2000;182(6):1580–91.
Felk A, Kretschmar M, Albrecht A, Schaller M, Beinhauer S, Nichterlein T, et al. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect Immun. 2002;70(7):3689–700.
Ernst JF. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology. 2000;146(8):1763.
Brown AJP, Gow NAR. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7(8):333–8.
Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16(8):1982–91.
Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90(5):939–49.
Noffz CS, Liedschulte V, Lengeler K, Ernst JF. Functional mapping of the Candida albicans Efg1 regulator. Eukaryot Cell. 2008;7(5):881–93.
Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7(11):1546–54.
Sonneborn A, Bockmuhl DP, Ernst JF. Chlamydospore formation in Candida albicans requires the Efg1p morphogenetic regulator. Infect Immun. 1999;67(10):5514–7.
Staib P, Kretschmar M, Nichterlein T, Hof H, Morschhauser J. Transcriptional regulators Cph1p and Efg1p mediate activation of the Candida albicans virulence gene SAP5 during infection. Infect Immun. 2002;70(2):921–7.
Korting HC, Hube B, Oberbauer S, Januschke E, Hamm G, Albrecht A, et al. Reduced expression of the hyphal-independent Candida albicans proteinase genes SAP1 and SAP3 in the efg1 mutant is associated with attenuated virulence during infection of oral epithelium. J Med Microbiol. 2003;52(Pt 8):623–32.
Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998;33(6):451–9.
Sharkey LL, McNemar MD, Saporito-Irwin SM, Sypherd PS, Fonzi WA. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J Bacteriol. 1999;181(17):5273–9.
Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, Cutler JE, et al. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002;44(1):61–72.
Nakayashiki H. RNA silencing in fungi: mechanisms and applications. FEBS Lett. 2005;579(26):5950–7.
Drinnenberg IA, Weinberg DE, Xie KT, Mower JP, Wolfe KH, Fink GR, et al. RNAi in budding yeast. Science. 2009;326(5952):544–50.
Harrison BR, Yazgan O, Krebs JE. Life without RNAi: noncoding RNAs and their functions in Saccharomyces cerevisiae. Biochem Cell Biol. 2009;87(5):767–79.
Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev. 2009;61(9):746–59.
Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457(7228):405–12.
Romano N, Macino G. Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol Microbiol. 1992;6(22):3343–53.
Rappleye CA, Engle JT, Goldman WE. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol Microbiol. 2004;53(1):153–65.
Nakayashiki H, Nguyen QB. RNA interference: roles in fungal biology. Curr Opin Microbiol. 2008;11(6):494–502.
Zhao W, Fanning ML, Lane T. Efficient RNAi-based gene family knockdown via set cover optimization. Artif Intell Med. 2005;35(1–2):61–73.
Liu H, Cottrell TR, Pierini LM, Goldman WE, Doering TL. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics. 2002;160(2):463–70.
Disney MD, Haidaris CG, Turner DH. Uptake and antifungal activity of oligonucleotides in Candida albicans. Proc Natl Acad Sci USA. 2003;100(4):1530–4.
Janbon G, Maeng S, Yang DH, Ko YJ, Jung KW, Moyrand F, et al. Characterizing the role of RNA silencing components in Cryptococcus neoformans. Fungal Genet Biol. 2010;47(12):1070–80.
Khatri M, Rajam MV. Targeting polyamines of Aspergillus nidulans by siRNA specific to fungal ornithine decarboxylase gene. Med Mycol. 2007;45(3):211–20.
Staab JF, White TC, Marr KA. Hairpin dsRNA does not trigger RNA interference in Candida albicans cells. Yeast. 2011;28(1):1–8.
De Backer MD, Nelissen B, Logghe M, Viaene J, Loonen I, Vandoninck S, et al. An antisense-based functional genomics approach for identification of genes critical for growth of Candida albicans. Nat Biotechnol. 2001;19(3):235–41.
Acknowledgments
The authors thank the Central Research Laboratory of the School of Public Health for providing laboratory facilities. This research was financially supported by a Tehran University of Medical Sciences (TUMS) grant.
Conflict of interest
The authors declare that there is no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moazeni, M., Khoramizadeh, M.R., Kordbacheh, P. et al. RNA-Mediated Gene Silencing in Candida albicans: Inhibition of Hyphae Formation by Use of RNAi Technology. Mycopathologia 174, 177–185 (2012). https://doi.org/10.1007/s11046-012-9539-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-012-9539-6