Abstract
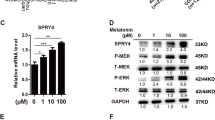
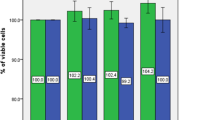
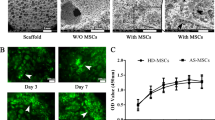
To investigate the role of mTOR signaling pathway in bone marrow mesenchymal stem cells (BMSCs) differentiation into osteoblast in degenerative scoliosis (DS). The rat model of DS was established. Thirty-two Sprague–Dawley (SD) rats were selected and divided into the normal control group, the positive control group (normal rats injected with rapamycin), the negative control group (DS rats injected with PBS) and the experiment group (DS rats injected with rapamycin). H&E staining was performed to observe the osteogenesis of scoliosis. The BMSCs were obtained and assigned into seven groups: the normal control group, the positive control group, the negative control group and 1.0/10.0/100.0/1000.0 nmol/L experiment groups. Flow cytometry was conducted to testify cell cycle. The mRNA and protein expressions of mTOR and osteoblastic differentiation markers were measured by qRT-PCR and western blotting. In vivo, compared with the negative control group, bone trabecular area and the number of differentiated bone cells were significantly increased in the experiment groups. In vitro, at 24 and 48 h after rapamycin treatment, compared with the negative control group, BMSCs at G0/G1 stage increased, but BMSCs at S stage decreased in the 1.0/10.0/100.0/1000.0 nmol/L experiment groups; the expressions of mTOR and p70-S6K1 proteins were reduced in the 1.0/10.0/100.0/1000.0 nmol/L experiment groups, while ALP activity, OC levels, calcium deposition, Co1-I protein expression and the mRNA expressions of OC and Co1-I were significantly increased. Suppression of mTOR signaling pathway by rapamycin could promote BMSCs differentiation into osteoblast in DS.







Similar content being viewed by others
References
Zheng J, Yang Y, Zhao K, Wang R (2015) Low expression of microRNA-143 is related to degenerative scoliosis possibly by regulation of cyclooxygenase-2 expression. Int J Clin Exp Med 8(3):4140–4145
Zhu Y, Wang K, Wang B, Wang H, Jin Z, Zhu Z, Liu H (2015) Selection of proximal fusion level for degenerative scoliosis and the entailing proximal-related late complications. Int J Clin Exp Med 8(4):5731–5738
Kotwal S, Pumberger M, Hughes A, Girardi F (2011) Degenerative scoliosis: a review. HSS J 7(3):257–264
Han S, Zhu Y, Wu Z, Zhang J, Qiu G (2013) The differently expressed proteins in MSCs of degenerative scoliosis. J Orthop Sci 18(6):885–892
Li D, Wang C, Chi C, Wang Y, Zhao J, Fang J, Pan J (2016) Bone marrow mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory reactions in macrophages and endothelial cells. Mediators Inflamm 2016:2631439
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12(1):21–35
Urbanska M, Gozdz A, Swiech LJ, Jaworski J (2012) Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J Biol Chem 287(36):30240–30256
Prasad G, Sottero T, Yang X, Mueller S, James CD, Weiss WA, Polley MY, Ozawa T, Berger MS, Aftab DT, Prados MD, Haas-Kogan DA (2011) Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with temozolomide. Neuro Oncol 13(4):384–392
Awasthi N, Yen PL, Schwarz MA, Schwarz RE (2012) The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J Cell Biochem 113(3):784–791
Huang X, Zeng Y, Wang X, Ma X, Li Q, Li N, Su H, Huang W (2016) FXR blocks the growth of liver cancer cells through inhibiting mTOR-s6K pathway. Biochem Biophys Res Commun 474(2):351–356
Zhang Z, Yang M, Wang Y, Wang L, Jin Z, Ding L, Zhang L, Zhang L, Jiang W, Gao G, Yang J, Lu B, Cao F, Hu T (2016) Autophagy regulates the apoptosis of bone marrow-derived mesenchymal stem cells under hypoxic condition via AMP-activated protein kinase/mammalian target of rapamycin pathway. Cell Biol Int 40(6):671–685
Gharibi B, Farzadi S, Ghuman M, Hughes FJ (2014) Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells 32(8):2256–2266
Yang C, Li Y, Zhao Y, Zhu X, Li M, Liu G (2016) Adult degenerative scoliosis: can cobb angle on a supine posteroanterior radiograph be used to predict the cobb angle in a standing position?. Medicine (Baltimore) 95(6):e2732
Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK (2002) Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 8(2):128–135
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE (2006) Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 10(2):159–170
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Tateda S, Yahata K, Itoi E (2012) The role of mTOR signaling pathway in spinal cord injury. Cell Cycle 11(17):3175–3179
Zheng N, Ding X, Jahan R (2014) Low concentration of rapamycin inhibits hemangioma endothelial cell proliferation, migration, and vascular tumor formation in mice. Curr Ther Res Clin Exp 76:99–103
Russo E, Andreozzi F, Iuliano R, Dattilo V, Procopio T, Fiume G, Mimmi S, Perrotti N, Citraro R, Sesti G, Constanti A, De Sarro G (2014) Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav Immun 42:157–168
Cho HJ, Park J, Lee HW, Lee YS, Kim JB (2004) Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem Biophys Res Commun 321(4):942–948
Vignot S, Faivre S, Aguirre D, Raymond E (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 16(4):525–537
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469(7330):323–335
Antonarakis ES, Carducci MA, Eisenberger MA (2010) Novel targeted therapeutics for metastatic castration-resistant prostate cancer. Cancer Lett 291(1):1–13
Brennan-Speranza TC, Conigrave AD (2015) Osteocalcin: an osteoblast-derived polypeptide hormone that modulates whole body energy metabolism. Calcif Tissue Int 96(1):1–10
Sun H, Kim JK, Mortensen R, Mutyaba LP, Hankenson KD, Krebsbach PH (2013) Osteoblast-targeted suppression of PPARgamma increases osteogenesis through activation of mTOR signaling. Stem Cells 31(10):2183–2192
Acknowledgements
The authors would like to thank the reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Wang, Y., Yi, XD. & Li, CD. Suppression of mTOR signaling pathway promotes bone marrow mesenchymal stem cells differentiation into osteoblast in degenerative scoliosis: in vivo and in vitro. Mol Biol Rep 44, 129–137 (2017). https://doi.org/10.1007/s11033-016-4089-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4089-5