Abstract
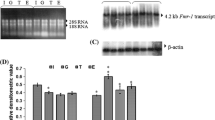
Fragile X mental retardation protein (FMRP) has been implicated in learning, memory and cognition, therefore, information on alterations in FMRP expression during maturation and aging may provide a clue towards understanding mechanisms of age-dependent cognitive changes in the brain. In the present paper, we have studied Fmr-1 gene expression and its correlation with interaction of a tans-acting factor Sp1with Fmr-1 promoter in the cerebral cortex of female mice at post natal period during maturation and aging. Our data reveal that level of Fmr-1 transcript in the cerebral cortex is significantly up regulated at day 7 after birth compared to day 0 (the day of birth) and is gradually down regulated from day 15 onward to old age. The pattern of Fmr-1 transcript levels corresponds with the level of FMRP, however, its level is significantly up regulated in old age compared to adult mice. Our EMSA data revealed the formation of a single complex as a result of binding of Sp1with Fmr-1 promoter sequence. Its intensity gradually decreased from the day 0 (day of birth) till day 15, remained unaltered in young, significantly decreased in adult and significantly increased in old age. Our data suggests that age-dependent alteration in the Fmr-1 gene expression is associated with Sp1 interaction with Fmr-1 promoter which in turn might be related with cognitive development during brain maturation and aging.



Similar content being viewed by others
References
Rosenzweig ES, Barnes CA (2003) Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69:143–179
Kreutz M, König I, Mikhaylova M, Spilker C, Zuschratter W (2008) Molecular Mechanisms of Dendritic Spine Plasticity in Development and Aging. Handbook of neurochemistry and molecular neurobiology: development and aging changes in the nervous system: 245–259
Lohmann C, Kessels HW (2014) The developmental stages of synaptic plasticity. J physiol 592:13–31
Huber KM, Kayser MS, Bear MF (2000) Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288:1254–1257
Sidorov MS, Auerbach BD, Bear MF (2013) Fragile X mental retardation protein and synaptic plasticity. Mol Brain 6:15
Scotto-Lomassese S, Nissant A, Mota T, Neant-Fery M, Oostra BA et al (2011) Fragile X mental retardation protein regulates new neuron differentiation in the adult olfactory bulb. J Neurosci 31:2205–2215
Siller SS, Broadie K (2011) Neural circuit architecture defects in a Drosophila model of Fragile X syndrome are alleviated by minocycline treatment and genetic removal of matrix metalloproteinase. Dis Model Mech 4:673–685
Friedman SH, Dani N, Rushton E, Broadie K (2013) Fragile X mental retardation protein regulates trans-synaptic signaling in Drosophila. Dis Model Mech 6:1400–1413
Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA et al (1997) Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci 94:5401–5404
Brackett DM, Qing F, Amieux PS, Sellers DL, Horner PJ et al (2013) FMR1 transcript isoforms: association with polyribosomes; regional and developmental expression in mouse brain. PLoS One 8:e58296
Eichler DC, Liberatore JA, Shumard CM (1993) Selection of a preribosomal RNA processing site by a nucleolar endoribonuclease involves formation of a stable complex. Nucleic Acids Res 21:5775–5781
Penagarikano O, Mulle JG, Warren ST (2007) The pathophysiology of fragile x syndrome. Annu Rev Genom Hum Genet 8:109–129
Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A et al (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146:247–261
Gross C, Berry-Kravis EM, Bassell GJ (2012) Therapeutic strategies in fragile X syndrome: dysregulated mGluR signaling and beyond. Neuropsychopharmacology 37:178–195
Bagni C, Tassone F, Neri G, Hagerman R (2012) Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Investig 122:4314–4322
Charalambous DC, Pasciuto E, Mercaldo V, Pilo Boyl P, Munck S et al (2013) KIF1Bbeta transports dendritically localized mRNPs in neurons and is recruited to synapses in an activity-dependent manner. Cell Mol Life Sci 70:335–356
Kalidas S, Smith DP (2003) Functional genomics, fragile X syndrome, and RNA interference. Arch Neurol 60:1197–1200
Gessert S, Bugner V, Tecza A, Pinker M, Kuhl M (2010) FMR1/FXR1 and the miRNA pathway are required for eye and neural crest development. Dev Biol 341:222–235
Gabus C, Mazroui R, Tremblay S, Khandjian EW, Darlix JL (2004) The fragile X mental retardation protein has nucleic acid chaperone properties. Nucleic Acids Res 32:2129–2137
De Rubeis S, Bagni C (2010) Fragile X mental retardation protein control of neuronal mRNA metabolism: insights into mRNA stability. Mol Cell Neurosci 43:43–50
Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S et al (2007) A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci 10:578–587
Peprah E (2012) Fragile X syndrome: the FMR1 CGG repeat distribution among world populations. Ann Hum Genet 76:178–191
Krueger DD, Bear MF (2011) Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med 62:411–429
De Rubeis S, Fernandez E, Buzzi A, Di Marino D, Bagni C (2012) Molecular and cellular aspects of mental retardation in the Fragile X syndrome: from gene mutation/s to spine dysmorphogenesis. Adv Exp Med Biol 970:517–551
Kumari D, Gabrielian A, Wheeler D, Usdin K (2005) The roles of Sp1, Sp3, USF1/USF2 and NRF-1 in the regulation and three-dimensional structure of the Fragile X mental retardation gene promoter. Biochem J 386:297–303
Smith KT, Nicholls RD, Reines D (2006) The gene encoding the fragile X RNA-binding protein is controlled by nuclear respiratory factor 2 and the CREB family of transcription factors. Nucleic Acids Res 34:1205–1215
Carrillo C, Cisneros B, Montanez C (1999) Sp1 and AP2 transcription factors are required for the human fragile mental retardation promoter activity in SK-N-SH neuronal cells. Neurosci Lett 276:149–152
Cote F, Schussler N, Boularand S, Peirotes A, Thevenot E et al (2002) Involvement of NF-Y and Sp1 in basal and cAMP-stimulated transcriptional activation of the tryptophan hydroxylase (TPH) gene in the pineal gland. J Neurochem 81:673–685
Smith KT, Coffee B, Reines D (2004) Occupancy and synergistic activation of the FMR1 promoter by Nrf-1 and Sp1 in vivo. Hum Mol Genet 13:1611–1621
Garber K, Smith KT, Reines D, Warren ST (2006) Transcription, translation and fragile X syndrome. Curr Opin Genet Dev 16:270–275
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Singh K, Gaur P, Prasad S (2007) Fragile x mental retardation (Fmr-1) gene expression is down regulated in brain of mice during aging. Mol Biol Rep 34:173–181
Gupta RK, Prasad S (2014) Differential regulation of GLT-1/EAAT2 gene expression by NF-kappaB and N-myc in male mouse brain during postnatal development. Neurochem Res 39:150–160
Dignam JD, Lebovitz RM, Roeder RG (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489
Prasad S, Singh K (2008) Interaction of USF1/USF2 and alpha-Pal/Nrf1 to Fmr-1 promoter increases in mouse brain during aging. Biochem Biophys Res Commun 376:347–351
Berman RF, Murray KD, Arque G, Hunsaker MR, Wenzel HJ (2012) Abnormal dendrite and spine morphology in primary visual cortex in the CGG knock-in mouse model of the fragile X premutation. Epilepsia 53(Suppl 1):150–160
Kaplan ES, Cao Z, Hulsizer S, Tassone F, Berman RF et al (2012) Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J Neurochem 123:613–621
Davidovic L, Navratil V, Bonaccorso CM, Catania MV, Bardoni B et al (2011) A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model. Genome Res 21:2190–2202
Lu R, Wang H, Liang Z, Ku L, O’Donnell WT et al (2004) The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA 101:15201–15206
Pan F, Aldridge GM, Greenough WT, Gan WB (2010) Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA 107:17768–17773
Pacey LK, Xuan IC, Guan S, Sussman D, Henkelman RM et al (2013) Delayed myelination in a mouse model of fragile X syndrome. Hum Mol Genet 22:3920–3930
Singh K, Prasad S (2008) Differential expression of Fmr-1 mRNA and FMRP in female mice brain during aging. Mol Biol Rep 35:677–684
Westmark CJ, Malter JS (2007) FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol 5:e52
Wang H, Wu LJ, Kim SS, Lee FJ, Gong B et al (2008) FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron 59:634–647
Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C et al (2007) Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 13:555–562
Drouin R, Angers M, Dallaire N, Rose TM, Khandjian EW et al (1997) Structural and functional characterization of the human FMR1 promoter reveals similarities with the hnRNP-A2 promoter region. Hum Mol Genet 6:2051–2060
Prasad S, Singh K (2008) Age- and sex-dependent differential interaction of nuclear trans-acting factors with Fmr-1 promoter in mice brain. Neurochem Res 33:1028–1035
Doetzlhofer A, Rotheneder H, Lagger G, Koranda M, Kurtev V et al (1999) Histone deacetylase 1 can repress transcription by binding to Sp1. Mol Cell Biol 19:5504–5511
Wu J, Xue L, Weng M, Sun Y, Zhang Z et al (2007) Sp1 is essential for p16 expression in human diploid fibroblasts during senescence. PLoS One 2:e164
Acknowledgments
PG thanks UGC for providing JRF and ICMR for Senior Research Fellowships (File No: 45/12/2008/BMS/aging). Financial assistance from CSIR (37/1389/09/EMR-II) and BRNS (2009/37/55/3298), Govt. of India to SP is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaur, P., Prasad, S. Alterations in the Sp1 binding and Fmr-1 gene expression in the cortex of the brain during maturation and aging of mouse. Mol Biol Rep 41, 6855–6863 (2014). https://doi.org/10.1007/s11033-014-3571-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3571-1