Abstract
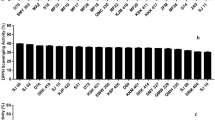
Oxidative stress is one of the major causes of degenerative conditions occurring at cellular level with serious health implications. This study was aimed at investigating the antioxidative potentials of probiotic lactobacilli of Indian gut origin and their ability to augment antioxidant defense enzyme systems in the host cells under oxidative stress conditions. A total of 39 Lactobacillus cultures were assessed for their resistance against reactive oxygen species. Most of the cultures were moderately to strongly resistant towards 0.4 mM H2O2. The Lactobacillus isolate CH4 was the most H2O2 resistant culture with only 0.06 log cycle reduction. Majority of the cultures demonstrated high resistance towards hydroxyl ions and Lp21 was the most resistant with log count reduction of 0.20 fold only. Almost all the cultures were also quite resistant to superoxide anions. Lp21 also showed the highest superoxide dismutase content (0.8971 U). Amongst the 39 cultures, Lactobacillus spp. S3 showed the highest total antioxidative activity of 77.85 ± 0.13 % followed by Lp55 (56.1 ± 1.2 %) in terms of per cent inhibition of linolenic acid oxidation. Lp9 up-regulated the expression of superoxide dismutase 2 gene in HT-29 cells both at 0.1 mM (1.997 folds) and 1.0 mM H2O2 (2.058 folds) concentrations. In case of glutathione peroxidase-1, Lp9, Lp91 and Lp55 showed significant (P < 0.001) up-regulation in the gene expression to the level of 5.451, 8.706 and 10.083 folds, respectively when HT-29 was challenged with 0.1 mM H2O2. The expression of catalase gene was also significantly up-regulated by all the cultures at 0.1 mM H2O2 conditions. It can be concluded that the antioxidative efficacy of the putative probiotic lactobacilli varied considerably between species and strains and the potential strains can be explored as prospective antioxidants to manage oxidative stress induced diseases.


Similar content being viewed by others
References
Marx JL (1985) Oxygen free radicals linked to many diseases. Science 235:529–531
Zommara M, Tachibana N, Sakono M et al (1996) Whey from cultured skim milk decrease serum cholesterol and increases antioxidant enzymes in liver and red blood cells in rats. Nutr Res 16:293–302
Oxman T, Shapira M, Diver A et al (2000) A new method of long-term preventive cardioprotection using Lactobacillus. Am J Physiol Heart Circ Physiol 278:H1717–H1724
Terahara M, Nishide S, Kaneko T (2000) Preventive effect of Lactobacillus delbrueckii subsp. bulgaricus on the oxidation of LDL. Biosci Biotechnol Biochem 64:1868–1873
Kullisaar T, Songisepp E, Mikelsaar M et al (2003) Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr 90:449–456
Kaizu H, Sasaki M, Nakajima H et al (1993) Effect of antioxidative lactic acid bacteria on rats fed a diet deficient in vitamin E. J Dairy Sci 76:2493–2499
Peuhkuri K, Lahteenmaki T, Sievi E et al (1996) Antioxidative properties of Lactobacillus GG measured as prostacyclin and nitric oxide production in endothelial cell culture. Nutr Today (Suppl) 31:53S–54S
Kaushik JK, Kumar A, Duary RK et al (2009) Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One 4(12):e8099. doi:10.1371/journal.pone.0008099
Kumar R, Grover S, Batish VK (2011) Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague–Dawley rats. Br J Nutr 105:561–573. doi:10.1017/S0007114510003740
Kumar R, Grover S, Batish VK (2011) Molecular identification and typing of putative probiotic indigenous Lactobacillus plantarum strain Lp91 of human origin by specific primed-PCR assays. Probiotics Antimicrob Prot 3:186–193. doi:10.1007/s12602-011-9083-6
Kullisaar T, Zilmer M, Mikelsaar M et al (2002) Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72:215–224
Barreto JC, Smith GS, Strobel NH et al (1995) Terephthalic acid: a dosimeter for the detection of hydroxyl radicals in vitro. Life Sci 56(4):PL89–PL96
Bauer AW, Kirby WMM, Sherris JC et al (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Pahhkla R, Zilmer M, Kullisaar T et al (1998) Comparison of the antioxidant activity of melatonin and pinoline in vitro. J Pineal Res 24:96–101
Starkopf J, Zilmer K, Vihalemm T et al (1995) Time course study of oxidative stress during open heart surgery. Scand J Thorac Cardiovasc Surg 29:181–186
Sauer J, Richter KK, Pool-Zobel BL (2007) Physiological concentrations of butyrate favorably modulate genes of oxidative and metabolic stress in primary human colon cells. J Nutr Biochem 18:736–745
Vanhoutvin SALW, Troost FJ, Hamer HM et al (2009) Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One 4:e6759. doi:10.1371/journal.pone.0006759
Lallemant B, Evrard A, Combescure C et al (2009) Reference gene selection for head and neck squamous cell carcinoma gene expression studies. BMC Mol Biol 10:78. doi:10.1186/1471-2199-10-78
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi:10.1373/clinchem.2008.112797
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RTPCR. Nucleic Acids Res 29:e45
Rasmussen R (2001) Quantification on the LightCycler. In: Meuer S, Wittwer C, Nakagawara K (eds) Rapid cycle real-time PCR, methods and applications. Springer Press, Heidelberg, pp 21–34
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Lee J, Hwang KT, Chung MY et al (2005) Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): role for a metal ion chelating effect. J Food Sci 70:M388–M391. doi:10.1111/j.1365-2621.2005.tb11524.x
Kim HS, Jeong SG, Ham JS et al (2006) Antioxidative and probiotic properties of Lactobacillus gasseri NLRI-312 isolated from Korean infant feces. Asian Aust J Anim Sci 19(9):1335–1341
De Freitas JM, Meneghini R (2001) Iron and its sensitive balance in the cell. Mutat Res 475:153–159
Gonzalez SN, Nadra-Chaud CA, Apella MC et al (1991) Evidence of superoxide dismutase in Lactobacillus acidophilus. Chem Pharm Bull 39:1065–1067
Yousten AA, Johnson JL, Salin M (1975) Oxygen metabolism of catalase-negative and catalase-positive strains of Lactobacillus plantarum. J Bacteriol 123:242–247
Tally FP, Goldin BR, Jacovus NV et al (1977) Superoxide dismutase in anaerobic bacteria of clinical significance. Infect Immun 16:20–25
Gregory EM, Fridovich L (1974) Oxygen metabolism in Lactobacillus plantarum. J Bacteriol 117:166–169
Gonzalez SN, Apella MC, Romero N et al (1989) SOD activity in some strains of lactobacilli: induction by manganese. Chem Pharm Bull 37:3026–3028
Rael LT, Thomas GW, Craun ML (2004) Lipid peroxidation and the thiobarbituric acid assay: standardization of the assay when using saturated and unsaturated fatty acids. J Biochem Mol Bio 37:749–752
Ou CC, Ko JL, Lin MY (2006) Antioxidative effects of intracellular extracts of yogurt bacteria on lipid peroxidation and intestine 407 cells. J Food Drug Anal 14(3):304–310
Lin MY, Yen CL (1999) Inhibition of lipid peroxidation by Lactobacillus acidophilus and Bifidobacterium longum. J Agric Food Chem 47:3661–3664
Zhang C, Li XY, Zhao L et al (2007) Lipopolysaccharide (LPS) upregulates the expression of heme oxygenase-1 in mouse placenta. Placenta 28:951–957
Hutt P, Shchepetova J, Loivukene K et al (2006) Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero and uropathogens. J Appl Microbiol 100:1324–1332
D’Souza A, Fordjour L, Ahmad A et al (2010) Effects of probiotics, prebiotics and synbiotics on messenger RNA expression of Caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula-fed neonatal rats. Ped Res 67:526–531
Yadav H, Jain S, Sinha PR (2008) Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res 75:189–195
Fabian E, Elmadfa I (2007) The effect of daily consumption of probiotic and conventional yoghurt on oxidant and anti-oxidant parameters in plasma of young healthy women. Int J Vitam Nutr Res 77:79–88
Chamari M, Djazayery A, Jalali M et al (2008) The effect of daily consumption of probiotic and conventional yogurt on some oxidative stress factors in plasma of young healthy women. ARYA Atheroscler J 4(4):175–179
Acknowledgments
We gratefully acknowledge the Director, National Dairy Research Institute (NDRI, Karnal, India) for providing facilities to carry out the study. The financial support received from National Dairy Research Institute (NDRI, India) and Indian Council of Agricultural Research (ICAR, India) in terms of providing fellowship to the first author of the paper to carry out her Master’s programme is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Achuthan, A.A., Duary, R.K., Madathil, A. et al. Antioxidative potential of lactobacilli isolated from the gut of Indian people. Mol Biol Rep 39, 7887–7897 (2012). https://doi.org/10.1007/s11033-012-1633-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1633-9