Abstract
TopBP1 protein displays structural as well as functional similarities to BRCA1 and is involved in DNA replication, DNA damage checkpoint response and transcriptional regulation. Aberrant expression of TopBP1 may lead to genomic instability and can have pathological consequences. In this study we aimed to investigate expression of TopBP1 gene at mRNA and protein level in hereditary breast cancer. Real-time quantitative PCR was performed in 127 breast cancer samples. Expression of TopBP1 mRNA in lobular carcinoma was significantly lower compared with ductal carcinoma (p < 0.05). The level of TopBP1 mRNA appeared to be lower in poorly differentiated (III grade) hereditary breast cancer in comparison with moderately (II grade) and well-differentiated cancer (I grade) (p < 0.05 and p < 0.001 respectively). We analyzed TopBP1 protein expression using immunohistochemistry and Western blot techniques. Expression of TopBP1 protein was found to be significantly increased in poorly differentiated breast cancer (III grade) (p < 0.05). The percentage of samples with cytoplasmic apart from nuclear staining increased with increasing histological grade. There was no significant association between level and intracellular localization of TopBP1 protein in hereditary breast cancer and other clinicopathological parameters such as estrogen and progesterone receptors status, appearance of metastasis in the axillary lymph nodes and type of cancer. Our data suggest that decreased level of TopBP1 mRNA and increased level of TopBP1 protein might be associated with progression of hereditary breast cancer.
Similar content being viewed by others
Introduction
TopBP1 protein (topoisomerase IIβ binding protein 1) was identified as an interacting partner for topoisomerase IIβ [1, 2]. TopBP1 possesses eight BRCA1 (breast cancer gene 1) carboxyl-terminal (BRCT) domains which were for the first time described for BRCA1 protein and are commonly found in proteins involved in regulation of the cell cycle checkpoint and the response of the cell to DNA damage [1, 3–5]. The C-terminal region of TopBP1 containing two BRCTs shows considerable similarity to the corresponding part of BRCA1. Beside the sequence homology, TopBP1 also displays functional similarities to BRCA1 [6, 7]. Both proteins are strongly induced during S phase. Following ionizing radiation, TopBP1 is recruited to DNA breaks and colocalizes with NBS1 (Nijmegen breakage syndrome protein 1), BRCA1, and 53BP1 (p53 binding protein 1) in nuclear foci [7–9]. TopBP1 interacts with several other proteins, including human papilliomavirus type 16 (HPV16) transcription/replication factor E2, DNA polymerase ε, checkpoint protein hRad9, and transcription factor E2F1 [8, 10–15]. Thus, TopBP1 seems to be involved in DNA replication, DNA damage checkpoint response and transcriptional regulation [3, 9, 13, 16–19]. TopBP1 protein is essential for maintenance of chromosomal integrity and cell proliferation. This protein appeared to be involved in DNA damage response, DNA replication checkpoint, chromosome replication and regulation of transcription [20–22]. TopBP1 knockout mouse exhibits early embryonic lethality at the peri-implantation stage and TopBP1 deficiency induces cellular senescence in primary cells [20, 22].
Genomic stability in eukaryotic cells is maintained by checkpoint mechanisms which coordinate cell cycle progression and other processes including transcription, apoptosis and DNA repair [3]. Some signals of DNA damage can lead to cell cycle arrest to prevent transfer of damaged genetic information to the daughter cells. Checkpoint responses are considered to be a major mechanism to reduce both initiation and progression of cancer, which can be caused by incomplete DNA repair, resulting in genetic alterations of tumor suppressor genes and protooncogenes [7]. TopBP1 appears to be directly involved in controlling replication initiation. In this regard, its role in repressing E2F1-mediated apoptosis at the G1/S transition would be crucial to ensure suppression of apoptosis before initiation of DNA replication. Like other proteins that are directly involved in DNA replication, TopBP1 is induced by E2F1 during G1/S transition. Therefore, TopBP1 acts as a critical cooperator to enforce the execution of S phase [3, 14, 17]. Both, TopBP1 and BRCA1 specifically regulate the G2/M checkpoint partially compensating each function [7]. At G2/M checkpoint, TopBP1 binds and activates ATR (ATM and Rad3-related protein) in an ATRIP (ATR interacting protein)-dependent manner, leading to recruitment and phosphorylation of BRCA1 and subsequent activation of Chk1 (checkpoint kinase 1) and other ATR effectors [23, 24]. Activation of Chk1 can lead to cell cycle arrest and damage repair [7, 14].
Aberrant expression of TopBP1 may be involved in the deregulation of processes controlled by this protein and can have pathological consequences. Therefore, the current study was aimed at investigating expression of TopBP1 gene at mRNA and protein level in hereditary breast cancers.
Materials and methods
Specimen collection
The studied material was obtained from Polish Mother’s Memorial Hospital, Research Institute, Lodz, Poland and comprised of 127 of formalin-fixed paraffin embedded (FFPE) sections of hereditary breast cancers (patients age range 28–69 years) and 24 normal breast tissues (patients age range 35–59 years). Apart from FFPE for 50 of 127 breast cancer patients fresh frozen (−80 °C) breast tissues were also obtained. Blood samples were obtained for each patient. Normal tissues were obtained from women undergoing radical mastectomy and after resection were immediately frozen at −80 °C. Cancerous and normal tissues belong to different persons.
Criteria for classifying the samples as hereditary breast cancers were as follows (1) at least one first-degree relative with breast cancer, regardless of age (n = 92), (2) breast cancer diagnosed below 40 years of age (n = 35). We analyzed three BRCA1 mutations (C61G, 4153delA, 5382insC) in DNA of studied breast cancer patients (see details in Method section). BRCA1 mutations were detected in 72 of 127 of studied patients. None of the patients received neoadjuvant endocrine therapy, chemotherapy or radiotherapy. A structured questionnaire was used to collect detailed information on age, age of menarche and/or menopause, weight, and family history of cancer. The pathological evaluation report was obtained for each patient (Table 1).
Analysis of BRCA1 mutations
Mutations of BRCA1 and BRCA2 genes confer a high lifetime risk for both breast and ovarian cancer. Many different BRCA1 and BRCA2 mutations have been described in families with early-onset breast and ovarian cancer [25–28]. In Poland three mutations (C61G, 4163delA and 5382insC) in BRCA1 gene account for 86 % of all BRCA1 and BRCA2 mutations [29, 30]. Mutations in BRCA2 are relatively rare in Poland and no founder BRCA2 mutations have been identified [29, 30]. Therefore, we analyzed presence of C61G, 4163delA and 5382insC in studied patients with breast cancer.
Genomic DNA was prepared from peripheral blood by using the commercial GenElute Blood Genomic DNA Kit (Sigma Aldrich, Germany) according to the manufacturer’s instruction. The allele specific oligonucleotides polymerase chain reaction (ASO-PCR) was used to determine the studied mutations of BRCA1 gene. PCR assays were performed in a total reaction volume of 25 μl containing 50 ng of genomic DNA, 1U Taq DNA polymerase (Sigma Aldrich, Germany), 1× reaction buffer (10 mM Trizma HCl, pH 8.3; 50 mM KCl; 1.5 mM MgCl2; 0.001 % (w/v) gelatin), 200 μM of each dNTP, 0.25 μM of each primer. PCR amplifications were conducted in GeneAmp PCR System 9700 (Perkin-Elmer, USA). Thermal cycling conditions were as follows: initial denaturation step at 94 °C for 5 min, 30 cycles at 95 °C for 30 s, 30 s at 54 °C for C61 > G, 51 °C for 4153delA and 60 °C for 5382insC, and 60 s at 72 °C. The terminal extension step was performed for 10 min at 72 °C. The following allele specific oligonucleotides as primers were used: F:5′GGTTTCTCAGATAACTGGGCC3′ (wild type variant), F:5′GGTTTCTCAG-ATAACTGGGCG3′ (mutated type variant) and R:5′CGTCAAAGAATACCCATCTG3′ (common primer) for C61G mutation; F:5′GGCATCTCAGGAACATCACC3′ (common primer), R:5′CTTGCCCGTTCCTCTTTCTTC3′ (wild type variant) and F:5′CTTGCCCGTTCCTCTTTCTGA3′ (mutated type variant) for 4153delA mutation; F:5′TGTTGGTCAGACTGGTGTCG3′ (common primer), F:CATTGACCAC-ATCTCCTCTGAC3′ (wild type variant) and F:5′CATTGACCACATCTCCTCTGGA3′ (mutated type variant) for 5382insC mutation. The PCR products (163, 220 and 225 bp) were separated onto 8 % polyacrylamide gel, stained with ethidium bromide and viewed under UV light.
Total RNA extraction from FFPE breast cancer sections and fresh frozen normal and cancerous breast tissues
Sections were deparaffinized by two rinses in xylene for 10 min at room temperature with shaking, followed by centrifugations at room temperature for 5 min at 12,000× g. After deparaffinization, we introduced a rehydratation step (rinsing in 100% ethanol, 85, 70 % ethanol, all prepared with diethylpyrocarbonate (DEPC) treated dH2O, for 5 min). The tissue was collected by centrifugation at 12,000× g for 5 min. After the final wash, alcohol was aspirated. The dried tissue pellets of breast cancer samples and fresh frozen normal and cancer breast tissues were resuspended in 500 μl of digestion buffer (10 mM NaCl; 500 mM Tris–HCl, pH 8.0; 25 mM EDTA; 1 % SDS) and 1 mg/ml proteinase K was added. Samples were incubated at 45 °C overnight. Prior to RNA purification, in same samples we inactivated proteinase K at 97 °C for 10 min. The digested samples were extracted using TRI Reagent (Sigma Aldrich, USA) according to manufacturer’s protocol. RNA was eluted in 20 μl RNase-free water, quantified by spectrophotometry at 260 nm and stored at −20 °C. RNA with a 260/280 nm ratio in range 1.8–2.0 was considered high quality. Integrity was evaluated by assessing the 18S and 28S rRNA bands in 1 % ethidium bromide stained agarose gels.
cDNA synthesis
First strand cDNA was synthesized from each RNA pool using PCR Kit ver 3.0 (Takara Bio Inc., Japan) according to the manufacturer’s instruction. Briefly, 1 μg RNA was combined with 2.5 pmol of oligo dT-adapter primer, 4 μl of 25 mM MgCl2, 2 μl 10× RNA PCR buffer, 2 μl of 10 mM dNTP mixture, 20 units of RNase inhibitor, 5 units of AMV reverse transcriptase XL, and RNase-free water to total volume of 20 μl. The reaction took place at 42 °C for 30 min, followed by 95 °C for 5 min and 5 °C for 5 min in a GeneAmp PCR System 9700 (Perkin-Elmer Co, USA). cDNA was stored at −20 °C.
Real-time quantitative PCR
The real-time PCR was performed in Mastercycler ep Realplex 4S (Eppendorf, Germany). Primers and TaqMan probes for TopBP1 and GAPDH control reference gene were designed and synthesized according to TaqMan Gene Expression Assay (assay Hs00199775_m1 and Hs00266705_g1, respectively) (Applied Biosystems, USA). PCR reactions were carried out in a total volume of 10 μl of universal master mix (Applied Biosystems, USA) and 1 μl cDNA. The reactions were performed in duplicate. A positive result was defined by a threshold cycle (Ct) value lower than 40 (the Ct value is determined by the number of cycles needed to exceed the background signal). Ct value of all positive results were lower than 30. Abundance of TopBP1 mRNA in samples was quantified by the ΔCt method. ΔCt (CtTopBP1 − CtGAPDH) values were recalculated into relative copy number values (number of copies of TopBP1 mRNA per 1,000 copies of GAPDH mRNA).
Sequencing of PCR products
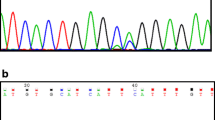
We amplified fragment of TopBP1 cDNA by PCR and purified products using QIAquick purification columns (Qiagen, USA). Both strands were sequenced using a BigDye Terminator Cycle Sequencing Kit ver. 1.1 (Applied Biosystems, USA) according to the manufacturer’s recommendations. Reactions were analysed on an ABI Prism 377 DNA Sequencer (Applied Biosystems, USA).
Immunohistochemistry
2 μm tissue sections were deparaffinized and rehydrated through xylene and graded ethanol, respectively. Slides were rinsed in dH2O, then were subjected to antigen retrieval in 10 mM citrate buffer, pH 6.0 by microwave oven heating for 20 min. Endogenous peroxidase activity was quenched in 0.3 % H2O2 for 30 min, then washed in dH2O. After blocking in Ready-to-Used Blocking Reagent (Bethyl, USA), slides were incubated 60 min at room temperature with rabbit anti-human TopBP1 antibody (Abcam, ab2402), dilution 1:200. Detection was performed with Immunohistochemistry Accessory Kit IHC-101 (Bethyl, USA) according to the performed by incubating the sections in a solution of DAB. Sections were counterstained with hematoxylin, dehydrated and coverslipped. For each antibody and samples a negative control was processed.
Evaluation of staining
Each case was evaluated in terms of staining intensity as positive and negative cells. The immunoreactive score (IRS) of the TopBP1 was estimated by multiplying the score for staining intensity (0—negative, 1—weak, 2—moderate and 3—strong staining) and score for the percentage of positively stained cells (0 = 0 %; 1 = <5 %; 2 = 5–35 %; 3 = >35–70 % and 4 = >70 %). An IRS score of 6 or higher was considered to be a strong reactivity, 4–5 moderate, 2–3 weak and 0–1 negative. The immunoreactive score results were estimated by two pathologists.
Western blot analysis
Cytoplasmic and nuclear fractions were separated from 50 cancerous and 24 normal fresh frozen breast tissue samples by differential centrifugation of tissue homogenate in isotonic sucrose in the presence of the serine protease inhibitor PMSF and 10 mM sodium molybdate. Nuclei were finally purified by centrifugation through 2.2 M sucrose. Protein concentration in homogenates and cellular fractions was evaluated according to Lowry protocol using bovine serum albumin (BSA) as standard. Proteins of homogenate as well as cytoplasmic and nuclear fractions (50 μg) were resolved on 8 % SDS-PAGE and electrotransferred onto Immobilon-P membrane (Millipore, USA). The loading and efficiency of transfer were verified by Ponceau S staining of membranes. After blocking in 0.5 % casein the membrane was incubated with specific primary anti-TopBP1 antibody diluted (1:5,000) in TBS with 0.5 % casein at 4 °C for 12 h. Commercial polyclonal antibody specific for the portion of human TopBP1 encoded within exon 28 was used (Abcam, UK). Following extensive washing with TBST buffer (Tris-buffered saline with Tween 20) the membrane was incubated with mouse anti-rabbit IgG-HRP antibody (Santa Cruz, USA). The specificity of antigen–antibody interaction was visualized with 4-chloro-1-naphthol and hydrogen peroxide as substrate for horseradish peroxidase (HRP). The intensities of the visualized signals were analyzed densitometrically.
Statistical analysis
Statistical analysis was performed using the STATISTICA version 9.0 (SatatSoft, Poland). Analysis of differences between TopBP1 mRNA and clinical parameters was carried out using the Mann–Whitney test and Kruskal–Wallis test with post hoc multiple comparisons. Relationships between TopBP1 protein expression and intracellular localization and clinicopathological factors were examined with Chi2 test. Analysis of the relationship between mRNA and protein level was performed using Spearman rank test. p value of p < 0.05 was considered statistically significant.
Results
The expression of TopBP1 gene at the mRNA level in normal breast tissue and hereditary breast cancer was estimated by real-time quantitative PCR analysis with GAPDH gene applied as a reference. Sequencing of PCR products confirmed that amplification product correspond with full-length sequence of TopBP1 and there were no other isoforms. In case of 50 cancer samples TopBP1 mRNA expression was analyzed both in FFPE sections and fresh breast tissues. Since there was no differences between mRNA expression in FFPE samples and fresh tissues only results from FFPE sections are presented on diagrams and table. The comparison of TopBP1 mRNA level with different clinicopathological parameters of tumors is shown in Table 2, where the average of TopBP1 mRNA copies per 1,000 copies of GAPDH mRNA represent the mean level of TopBP1 mRNA, and are used for statistical analysis. TopBP1 mRNA expression was observed in all of 24 (100 %) normal breast tissue samples and in 97 of 127 (76.4 %) breast cancer samples. Thus, positive expression of TopBP1 mRNA in normal tissue was more frequently observed compare to breast cancer samples (p < 0.05). Level of TopBP1 mRNA was significantly higher in normal breast tissues than in breast cancer specimens (p < 0.01; Fig. 1a). Expression of TopBP1 mRNA was found to be significantly decreased in the lobular carcinoma compared to the ductal carcinoma (p < 0.05; Fig. 1b). Fifteen of 28 (53.6 %) lobular carcinoma demonstrated detectable mRNA for TopBP1 gene, while the expression of TopBP1 mRNA was observed in 76 of 99 (78.3 %) ductal carcinoma. Expression of TopBP1 gene at the mRNA level was observed in 62.0 % (23/37) of poorly differentiated breast cancer (grade III), whereas nearly 80 % of tumors in I and II grade demonstrated detectable TopBP1 mRNA (78.6 % (11/14) and 78.9 % (60/76), respectively). Significantly lower TopBP1 mRNA level in the poorly differentiated (grade III) familial breast cancer compared with moderately (grade II, p < 0.01) and well-differentiated cancer (grade I, p < 0.001) was noted (Fig. 1c). There was no statistically significant difference in the context to clinicopathological parameters such as estrogen and progesterone receptor status and appearance of metastasis in the axillary lymph nodes.
The expression level of TopBP1 protein was determined in 127 breast carcinoma samples using immunohistochemical technique. The results concerning correlation between TopBP1 expression and clinicopathological parameters of breast cancers are shown in Fig. 2 and Table 3. TopBP1 was expressed in 121 of 127 (95.3 %) breast cancers. In 80/121 (66.1 %) there were heterogenous immunoreactivity with areas of nuclear and cytoplasmic staining (4 cases in grade I, 52 cases in grade II and 24 cases in grade III). Of the 121 cases, 51 (40.1 %) had a weak expression of TopBP1 protein (IRS score 2–3), 61 (48.0 %) a moderate expression (IRS score 4–5) and 9 (7.1 %) a strong expression (IRS score ≥6). Pathological grading was associated with TopBP1 protein expression. All tumor samples classified as grade I and grade II showed TopBP1 protein expression. In grade I, 12 cases had a weak and 2 a moderate expression and 0 cases strong TopBP1 protein expression. Six of 37 samples characterized as grade III had a negative TopBP1 protein expression, whereas 4 had a weak, 22 a moderate, and 5 a strong TopBP1 protein expression. There was no expression difference between type of tumor, lymph node status, and steroid hormone receptors status (Fig. 2; Table 3).
Expression of TopBP1 protein was also examined using Western blot, in both normal breast tissue and carcinoma specimens in 24 and 50 cases, respectively. The results concerning TopBP1 expression in homogenates and its cellular localization are shown in Fig. 3c. Among 50 analyzed tumors, 4 were classified as grade I, 30 as grade II and 16 as grade III. The mean level of TopBP1 in homogenates was higher in cancer samples compared to normal cells (p < 0.05). Moreover, we observed higher TopBP1 protein level in the poorly differentiated breast cancers compared to moderately and well-differentiated cancers (Fig. 3a, b). These results are consisted with the results obtained with immunohistochemistry method (Fig. 2).
Western blot analysis of TopBP1 protein expression and localization. a TopBP1 protein expression in homogenate samples of normal and cancer breast tissue (sample no. 15 was used as a reference sample), b results of densitometric analysis of the intensity of TopBP1 bands in normal and cancer breast samples, and in relation to tumor grade, c nuclear and cytoplasmic localization of TopBP1 in representative normal and breast cancer tissues
TopBP1 protein was found in the nuclear fraction of all normal breast tissue samples. Low level of TopBP1 was also detected in cytoplasmic fraction of seven normal tissue specimens. TopBP1 protein was found in all nuclear and one cytoplasmic fraction of analyzed breast cancer in grade I. Pattern of TopBP1 protein expression in well-differentiated breast cancer was similar to normal breast tissue samples. In 10 of 30 moderate differentiated breast cancers expression of TopBP1 was observed only in the nuclear fraction. In the remaining 20 tumor samples in grade II, TopBP1 protein was also detected in cytoplasmic fraction. However, level of this protein was lower in cytoplasmic fraction than nuclear fraction. In the case of four poorly differentiated breast cancer, TopBP1 protein was in the nuclear fraction and was absent in the cytoplasmic fraction. In 6 of 16 breast cancer in grade III, TopBP1 protein was reveals in both the nuclear and cytoplasmic fractions. However, the level of TopBP1 protein in two of these samples was higher in cytoplasmic fraction than in the nuclear fraction.
The results of TopBP1 protein expression showing higher TopBP1 level in cancer samples compared to normal tissues are in contrast to the results concerning mRNA level of TopBP1. In most cases of tumors we observed lower level of mRNA level than in normal tissue. Moreover, in most cases of tumors we found an inverse correlation between protein and mRNA level (Spearman’s rank analysis, R = −0.61, p > 0.05).
Discussion
Breast cancer is the commonest malignancy in women and it is estimated that a million women worldwide will develop breast cancer each year [31]. About 5–10 % of the cases are considered familial [6]. A number of high penetrance breast cancer susceptibility genes have been identified and include BRCA1 and BRCA2. These genes confer a high risk of breast and ovarian carcinoma. About 40–50 % of familial breast cancer can currently be explained by mutations in BRCA1 and BRCA2 genes. Of the remaining cases no more than 5 % are caused by defects in other studied genes, such as p53, PTEN, ATM and Chk2 [6, 32, 33]. It is not known how many more genes that confer a small risk are yet to be identified or how these genes come together or interact with each other or with environmental factors to increase to breast cancer risk [31]. Most of the known cancer susceptibility genes encode proteins involved in the monitoring of genome integrity. Therefore, Karppinen et al. [6] suggested, that TopBP1 coding for protein displaying structural and functional similarities with BRCA1 can be a plausible susceptibility gene for hereditary breast and ovarian cancer.
In this study we examined the relationship between expression of TopBP1 gene at the mRNA and protein level and clinicopathological parameters of hereditary breast cancers. In the literature there is no data concerning expression of TopBP1 at mRNA level in normal and cancerous tissues and only a few studies have examined the expression of TopBP1 protein in female breast cancer [34–37].
Our results obtained by immunohistochemical and Western blot analyses showed higher level of TopBP1 protein in breast cancer samples compared with normal breast tissues. Moreover, patients with overexpression of TopBP1 tend to have higher grades of breast cancer than those without overexpression of TopBP1. These results are consistent with results of Liu et al. [37] who have also showed higher expression of TopBP1 in breast cancers than normal tissues and found that patients with overexpression of TopBP1 in the tumors have significantly shorter overall survival time and shorter progression free survival time than those without overexpression of TopBP1. Therefore, TopBP1 may be an important prognostic marker for aggressive subgroups of breast cancer [37]. Since TopBP1 is involved in regulation of promoter binding activity of p53 during normal growth, the authors suggest that, its abnormally high level may potentially inactivate p53 and contribute to an aggressive behavior of breast tumors [37].
Going and coworkers [34] who analyzed the TopBP1 expression by immunohistochemistry in normal and cancerous breast tissues reported the significant changes in TopBP1 localization. In normal breast tissue, TopBP1 staining was almost entirely nuclear in location, although in rare cells some cytoplasmic staining was occasionally detected [34]. The nuclear localization is consistent with the function of TopBP1 which is involved in DNA damage response and initiation of DNA replication [4, 15]. However, the pattern of TopBP1 staining in human breast tissues changed from predominantly nuclear in normal epithelium, to nuclear and cytoplasmic or purely cytoplasmic in most of cancers [34]. Expression of TopBP1 was also reported in feline and canine mammary neoplasia [35, 36]. TopBP1 staining was predominantly nuclear, but in some tumors there was additional cytoplasmic staining [35, 36]. Expression of TopBP1 protein in feline and canine mammary neoplasia was positively correlated with histological grade and additionally to nuclear, cytoplasmic staining was observed as the degree of malignancy increased [34–36]. Our results concerning localization of TopBP1 are consistent with results ascribed as above. We also found in normal tissue mainly nuclear localization of TopBP1 whereas in most cancer tissue samples TopBP1 localized also in cytoplasmic compartment. The percentage of samples with cytoplasmic expression of TopBP1 increased with increasing histological grade. However, there was no significant association between level and intracellular localization of TopBP1 protein in hereditary breast cancer and other clinicopathological parameters as estrogen and progesterone receptors status, appearance of metastasis in the axillary lymph nodes and type of cancer.
There are no studies that demonstrated expression of TopBP1 at mRNA level and its relationship to TopBP1 protein level in normal and cancerous breast tissues. The results of our present study surprisingly showed lower level of TopBP1 mRNA in cancer samples compared to normal tissues. We also found that expression of TopBP1 mRNA contrary to TopBP1 protein level was inversely correlated with histological grade of hereditary breast cancers. Moreover, expression of TopBP1 mRNA was significantly down-regulated in the lobular carcinoma compared to the ductal carcinoma. However, we did not find any correlation between TopBP1 protein level and type of breast cancer.
At present we do not know the reason of discrepancies between TopBP1 mRNA and protein level. The lack of compatibility between alteration in mRNA and protein levels confirms that there is no simple and direct relation between transcriptom and proteome. Many factors may influence on the protein content in the cells. We suggest that in regulation of TopBP1 mRNA and protein levels a kind of negative feedback control mechanism may be involved. Down-regulation of mRNA concurrent with up-regulation of protein level may occur when a protein half-life is increased due to stabilization because components involved in the protein’s normal turnover may be disrupted or the protein may be stabilized through protein–protein interactions. There is possibility that in cancer cells TopBP1 is more stable and can be accumulated. We do not know what could make TopBP1 more stable but it can be associated with TopBP1 mislocalization in cancer cells. Accumulated TopBP1 may act as a repressor of transcription. Our results showing inverse correlation between TopBP1 mRNA and protein levels in breast cancers seem to confirm this hypothesis. Expression of TopBP1 gene is regulated by E2F1 and Egr-1 [38, 39]. On the other hand TopBP1 regulates activity of E2F1 and high level of this protein suppresses E2F1 transcriptional activity without affecting E2F1 protein levels [8, 14]. Thus, decreased transcriptional activity of E2F1 by TopBP1 may repress the expression of E2F1 target genes, including TopBP1.
In summary, the association between TopBP1 mRNA and protein expression and aggressive behavior of breast cancer could have a potential therapeutic implication. However, mechanisms of TopBP1 expression regulation need to be elucidated.
References
Wright RHG, Dorman ES, Donaldson MM, Morgan IM (2006) TopBP1 contains a transcriptional activation domain suppressed by two adjacent BRCT domains. Biochem J 400:573–582
Yamane K, Kawabat M, Tsuruo T (1997) A DNA-topoisomerase-II-binding protein with eight repeating regions similar to DNA-repair enzymes and to a cell-cycle regulator. Eur J Biochem 256:794–799
Kim JE, McAvoy AA, Smith DI, Chen J (2005) Human TopBP1 ensures genome integrity during normal S phase. Mol Cell Biol 25:10907–10915
Yamane K, Tsuruo T (1999) Conserved BRCT regions of TopBP1 and of the tumor suppressor BRCA1 bind strand breaks and termini of DNA. Oncogene 18:5194–5203
Cescutti R, Negrini S, Kohzaki M, Halazonetis TD (2010) TopBP1 functions with 53BP1 in the G1 DNA damage checkpoint. EMBO J 29:3723–3732
Karppinen SM, Erkko H, Reini K, Pospiech H, Heikkinen K, Rapakko K, Syvaoja JE, Winqvist R (2006) Identification of a common polymorphism in the TopBP1 gene associated with hereditary susceptibility to breast and ovarian cancer. Eur J Cancer 42:2647–2652
Yamane K, Chen J, Kinsella TJ (2003) Both DNA topoisomerase II-binding protein 1 and BRCA1 regulate the G2-M cell cycle checkpoint. Cancer Res 63:3049–3053
Liu K, Lin FT, Ruppert JM, Lin WC (2003) Regulation of E2F1 by BRCT domain-containing protein TopBP1. Mol Cell Biol 23:3287–3304
Morishima KI, Sakamoto S, Kobayashi J, Izumi H, Suda T, Matsumoto Y, Tauchi H, Ide H, Komatsu K, Matsuura S (2007) TopBP1 associates with NBS1 and is involved in homologous recombination repair. Biochem Biophys Res Commun 362:872–879
Boner W, Taylor ER, Tsirimonaki E, Yamane K, Campo MS, Morgan IM (2002) A functional interaction between the human papillomavirus 16 transcription/replication factor E2 and the DNA damage response protein TopBP1. J Biol Chem 277:22297–22303
Donaldson MM, Boner W, Morgan IM (2007) TopBP1 regulates human papillomavirus type 16 E2 interaction with chromatin. J Virol 81:4338–4342
Herold S, Hock A, Herkert B, Berns K, Mullenders J, Beijersbergen R, Bernards R, Eilers M (2008) Miz1 and HectH9 regulate the stability of the checkpoint protein, TopBP1. EMBO J 27:2851–2861
Liu K, Paik JC, Wang B, Lin FT, Lin WC (2006) Regulation of TopBP1 oligomerization by Akt/PKB for cell survival. EMBO J 25:4795–4807
Liu K, Luo Y, Lin FT, Lin WC (2004) TopBP1 recruits BRG1/BRM to repress E2F1-induced apoptosis, a novel pRb-independent and E2F1-specific control for cell survival. Genes Dev 18:673–686
Makiniemi M, Hillukkala T, Tuusa J, Reini K, Vaara M, Huang D, Pospiech H, Majuri I, Westerling T, Makela TP, Syvaoja JE (2001) BRCT domain-containing protein TopBP1 functions in DNA replication and damage response. J Biol Chem 276:30099–30406
Delacroix S, Wagner JM, Kobayashi M, Yamamoto KI, Karnitx LM (2007) The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev 21:1472–1477
Jeon Y, Lee KY, Ko MJ, Lee YS, Kang S, Hwang DS (2007) Human TopBP1 participates in cyclin E/CDK2 activation and preinitiation complex assembly during G1/S transition. J Biol Chem 282:14882–14890
Kumagai A, Lee J, Yoo HY, Dunphy WG (2006) TopBP1 activates the ATR-ATRIP complex. Cell 124:943–955
Schmidt U, Wollmann Y, Franke C, Grosse F, Saluz HP, Hanel F (2008) Characterization of the interaction between the human DNA topoisomerase IIβ-binding protein 1 (TopBP1) and the cell division cycle protein 45 (Cdc45). Biochem J 409:169–177
Bang SW, Ko MJ, Kang S, Kim GS, Kang D, Lee JH, Hwang DS (2011) Human TopBP1 localization to the mitotic centrosome mediates mitotic progression. Exp Cell Res 317:994–1004
Garcia V, Furuya K, Carr AM (2005) Identification and functional analysis of TopBP1 and its homologs. DNA Repair 4:1227–1239
Jeon Y, Ko E, Lee KY, Ko MJ, Park SY, Kang J, Jeon CH, Lee H, Hwang DS (2011) TopBP1 deficiency causes an early embryonic lethality and induces cellular senescence in primary cells. J Biol Chem 286:5414–5422
Bucher N, Britten CD (2008) G2 checkpoint abrogation and checkpoint kinase-I targeting in the treatment of cancer. Br J Cancer 98:523–528
Cimprich KA, Cortez D (2008) ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 9:616–627
Gorski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, Pluzanska A, Bebenek M, Sischer-Maliszewska L, Grzybowska E, Narod SA, Lubinski J (2000) Funder mutations in the BRCA1 gene in polish families with breast-ovarian cancer. Am J Hum Genet 66:1963–1968
Mahfoudh W, Bouaouina N, Ahmed SB, Gabbouj S, Shan J, Mathew R, Uhrhammer N, Bignon YJ, Troudi W, Elgaaied ABA, Hassen E, Chouchane L (2011) Hereditary breast cancer in middle Eastern and north African (MENA) populations: identification of novel, recurrent and founder BRCA1 mutations in the Tunisian population. Mol Biol Rep. doi:10.1007/s11033-011-0829-8
Wang F, Fang Q, Ge Z, Yu N, Xu S, Fan X (2011) Common BRCA1 and BRCA2 mutations in breast cancer families: a meta-analysis from systematic review. Mol Biol Rep. doi:10.1007/s11033-011-0958-0
Xu J, Wang B, Zhang Y, Li R, Wang Y, Zhang S (2011) Clinical implication for BRCA gene mutation in breast cancer. Mol Biol Rep. doi:10.1007/s11033-011-1073-y
Gorski B, Cybulski C, Huzarski T, Byrski T, Gronwald J, Jakubowska A, Stawicka M, Gozdecka-Grodecka S, Szwiec M, Urbanski K, Mitus J, Marczyk E, Dziuba J, Wandzel P, Surdyka D, Haus O, Janiszewska H, Debniak T, Toloczko-Grabarek A, Medrek K, Masojc B, Mierzejewski M, Kowalska E, Narod SA, Lubinski J (2005) Breast cancer predisposing alleles in Poland. Breast Cancer Res Treat 92:19–24
Gorski B (2006) Selected aspects of molecular diagnostics of constitutional alterations in BRCA1 and BRCA2 genes associated with increased risk of breast cancer in the polish population. Hered Cancer Clin Pract 4:142–152
Da Silva L, Lakhani SR (2010) Pathology of hereditary breast cancer. Mod Pathol 23:S46–S51
Easton DF (1999) How many more breast cancer predisposition gene are there? Breast Cancer Res 1:14–17
Oldenburg RA, Meijers-Heijboer H, Cornelisse CJ, Devilee P (2007) Genetic susceptibility for breast cancer: how many more genes to be found? Crit Rev Oncol Hematol 63:125–149
Going JJ, Nixon C, Dorman ES, Boner W, Donaldson MM, Morgan IM (2007) Aberrant expression of TopBP1 in breast cancer. Histopathology 50:418–424
Morris JS, Nixon C, Bruck A, Nasir L, Morgan IM, Philbey AW (2008) Immunohistochemical expression of TopBP1 in feline mammary neoplasia in relation to histological grade Ki67, ERα and p53. Vet J 175:218–226
Morris JS, Nixon C, King OJA, Morgan IM, Philbey AW (2009) Expression of TopBP1 in canine mammary neoplasia in relation to histological type, Ki67, ERα and p53. Vet J 179:422–429
Liu K, Bellam N, Lin HY, Wang B, Stockard CR, Geizzle WE, Lin WC (2009) Regulation of p53 by TopBP1: a potential mechanism for p53 inactivation in cancer. Mol Cell Biol 29:2673–2693
Yoshida K, Inoue I (2004) Expression of MCM10 and TopBP1 is regulated by cell proliferation and UV irradiation via the E2F1 transcription factor. Oncogene 23:6250–6260
Usskilat C, Skerka C, Saluz HP, Hanel F (2006) The transcription factor EGR-1 is a regulator of the human TopBP1 gene. Gene 380:144–150
Acknowledgments
This work was supported by the grant of Integrated Operational Program for Regional Development, ZPORR (Grant No. 10/S-UŁ/2009). Ewa Forma is a recipient of D-RIM 1st edition fellowship co-funded by the European Union under the European Social Fund, POKL “Human-Best Investment!”.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Forma, E., Krzeslak, A., Bernaciak, M. et al. Expression of TopBP1 in hereditary breast cancer. Mol Biol Rep 39, 7795–7804 (2012). https://doi.org/10.1007/s11033-012-1622-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1622-z