Abstract
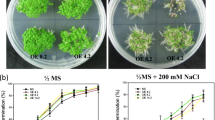
Dehydrins are capable of conferring abiotic stress tolerance in plants. Few dehydrins have been characterized at the molecular and transgenic level in Brassica juncea. In the present study, two SK2-type B. juncea dehydrin genes, BjDHN2 and BjDHN3, showed improved stress tolerance to salt and freezing stress in transgenic yeast. Semiquantitative reverse transcription polymerase chain reaction (RT-PCR) revealed that BjDHN2 and BjDHN3 were up-regulated by low temperature, drought, and salt stress, as well as exogenous abscisic acid (ABA) in B. juncea. Transgenic studies also showed that BjDHN2 and BjDHN3 increased tolerance to cold and salt stress in tobacco. These results indicate that the BjDHN2 and BjDHN3 genes exert a protective role on membranes and play a role in abiotic stresses.



Similar content being viewed by others
References
Alonso A, Queiroz CS, Magalhaes AC (1997) Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L.) seedlings. Biochim Biophys Acta 1323:75–84
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Barclay KD, McKersie BD (1994) Peroxidation reactions in plant membranes: effects of free fatty acids. Lipids 29:877–883
Cheng Z, Targolli J, Huang X, Wu R (2002) Wheat LEA genes, PMA80 and PMA1959, enhance dehydration tolerance of transgenic rice (Oryza sativa L.). Mol Breed 10:71–82
Close TJ (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Plant Physiol 100:291–296
Davidson WS, Jonas A, Clayton DF, George JM (1998) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273:9443–9449
Gilmour SJ, Artus NN, Thomashow MF (1992) cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol 18:13–21
Ginger A, Swire-Clark WR, Marcotte JR (1999) The wheat LEA protein Em functions as an osmoprotective molecule in Saccharomyces cerevisial. Plant Mol Biol 39:117–128
Hara M, Terashim S, Kuboi T (2001) Characterization and cryoprotective activity of cold-responsive dehydrin from Citrus unshiu. J Plant Physiol 158:1333–1339
Hara M, Terashima S, Fukaya T, Kuboi T (2003) Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 17:290–298
Hong Z, Lakkineni K, Zhang Z, Verma DPS (2000) Removal of feedback inhibition of D1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K (1994) Characterization of two cDNAs (ERD10 and ERD14) corresponding to genes that respond rapidly to dehydration stress in Arabidopsis thaliana. Plant Cell Physiol 35:225–231
Lång V, Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20:951–962
Li BL, Mei HS (1989) Relationship between oat leaf senescence and activated oxygen metabolism. Acta Phytophysiol Sinica 15:6–12
Li L, Staden JV (1998) Effects of plant growth regulators on drought resistance of two maize cultivars. S Afr J Bot 64:116–120
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nylander M, Svensson J, Palva ET, Welin BV (2001) Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol Biol 45:263–279
Rorat T (2006) Plant dehydrins – tissue location, structure and function. Cell Mol Biol Lett 11:536–556
Rouse D, Gehring CA, Parish RW (1992) Structure and sequence of a dehydrin-like gene in Arabidopsis thaliana. Plant Mol Biol 19:531–532
Svensson J, Palva ET, Welin B (2000) Purification of recombinant Arabidopsis thaliana dehydrins by metal ion affinity chromatography. Protien Exp Purif 20:169–178
Sunkar R, Bartels D, Kirch HH (2003) Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J 35:452–464
Thomashow MF (1998) Role of cold-responsive genes in plant freezing tolerance. Plant Physiol 118:1–7
Thomashow MF (1999) Plant cold acclimation, freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol 50:571–599
Welin BV, Olson Å, Nylander M, Palva ET (1994) Characterization and differential expression of dhn/lea/rab-like genes during cold acclimation and drought stress in Arabidopsis thaliana. Plant Mol Biol 26:131–144
Yan SP, Zhang QY, Tang ZC, Su W A, Sun WN (2006) Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteomics 5:484–496
Yao KN, Lockhart KM, Kalanack JJ (2005) Cloning of dehydrin coding sequences from Brassica juncea and Brassica napus and their low temperature-inducible expression in germinating seeds. Plant Physiol Biochem 43:83–89
Yu JN, Zhang JS, Shan L, Chen SY (2005) Two new group 3 LEA genes of wheat and their functional analysis in yeast. J Integr Plant Biol 47:1372–1381
Zhang L, Ohta A, Takagi M, Imai R (2000) Expression of plant group 2 and group 3 lea genes in Saccharomyces cerevisiae revealed functional divergence among LEA proteins. J Biochem 127:611–616
Zhang YX, Xu J, Han L, Wei W, Guan ZQ, Cong L, Chai TY (2006a) Highly efficient shoot regeneration and Agrobacterium-mediated transformation protocol of Brassica juncea. Plant Mol Biol Rep 24:255a–255i
Zhang YX, Li JM, Yu F, Cong L, Chai TY (2006b) Cloning and expression analysis of SKn-type dehydrin gene from bean in response to heavy metals. Mol Biotechnol 32:205–218
Zhang YX, Wang Z, Xu J (2007) Molecular mechanism of dehydrin in response to environmental stress in plant. Prog Nat Sci 17:237–246
Acknowledgments
The authors would like to thank Prof. Wenjun Ding of the Graduate University of Chinese Academy of Sciences for his critical review of the English manuscript. The research was supported by the National High Technology Planning Program of China (grant nos. 2006AA10Z407 and 2006AA06Z355), and the China National Natural Sciences Foundation (grant no. 30570146).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Zhang, Y., Guan, Z. et al. Expression and function of two dehydrins under environmental stresses in Brassica juncea L.. Mol Breeding 21, 431–438 (2008). https://doi.org/10.1007/s11032-007-9143-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-007-9143-5