Abstract
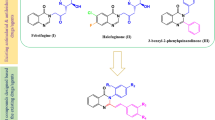
The quinazolin-2,4-dione moiety is found in many compounds with important biological activities making it a target for its synthesis. In this work, a one-pot three-step synthesis of new quinazolin-2,4-diones from phthalic anhydrides and their activity against Leishmania mexicana are described. The new quinazolin-2,4-diones were isolated with yields in the range of 32–70 %. All compounds displayed lower cytotoxicity in RAW 264.7 macrophage over miltefosine. Compound 6,7-dichloro-3-phenylquinazoline-2,4(1H,3H)-dione (6e) displayed an attractive profile which includes anti-Leishmania mexicana activity (\(\hbox {IC}_{50} = 6.05\) \(\upmu \)M), much lower cytotoxic activity (\(\hbox {CC}_{50} = 111\) \(\upmu \)M) and a high selective index (\(\text {SI} = 18.35\)) proving to be superior to miltefosine.


Similar content being viewed by others
References
Smith AL, Thomson CG, Leeson PD (1996) An efficient solid phase synthetic route to 1,3-disubstituted 2,4(1H,3H)-quinazolinediones suitable for combinatorial synthesis. Bioorg Med Chem Lett 6:1483–1486. doi:10.1016/s0960-894x(96)00253-3
Brogden RN, Sorkin EM (1990) Ketanserin. Drugs 40:903–949. doi:10.2165/00003495-199040060-00010
Hayao S, Havera HJ, Strycker WG, Leipzig TJ, Kulp RA, Hartzler HE (1965) New sedative and hypotensive 3-substituted 2,4(1H,3H)-quinazolinediones. J Med Chem 8:807–811. doi:10.1021/jm00330a017
Hassan MA, Younes AMM, Taha MM, Abdel-Monsef A-BH (2011) Synthesis and reactions of 3-aminotetrachloroquinazolin-2,4-dione. Eur J Chem 2:514–518. doi:10.5155/eurjchem.2.4.514-518.479
Hassan MA, Seleem MA, Younes AMM, Taha MM, Abdel-Monsef A-BH (2013) Synthesis and spectral characterization of some heterocyclic nitrogen compounds. Eur J Chem 4:121–123. doi:10.5155/eurjchem.4.2.121-123.740
Choo HY, Kim M, Lee SK, Kim SW, Chung IK (2002) Solid-phase combinatorial synthesis and cytotoxicity of 3-aryl-2,4-quinazolinediones. Bioorg Med Chem 10:517–523. doi:10.1016/S0968-0896(01)00299-1
Li Z, Huang H, Sun H, Jiang H, Liu H (2008) Microwave-assisted efficient and convenient synthesis of 2,4(1H,3H)-quinazolinediones and 2-thioxoquinazolines. J Comb Chem 10:484–486. doi:10.1021/cc800040z
Yalysheva NZ, Granik VG (1984) Unexpected formation of 2,4-quinazolinedione in the reaction of \(\alpha \)-cyano-\(\beta \)-dimethylaminocrotonamide with ethyl anthranilate. Chem Heterocycl Compd 20:1186–1186. doi:10.1007/BF00503620
Rayat S, Qian M, Glaser R (2005) Nitrosative cytosine deamination. An exploration of the chemistry emanating from deamination with pyrimidine ring-opening. Chem Res Toxicol 18:1211–1218. doi:10.1021/tx050082a
Gao J, He L-N, Miao C-X, Chanfreau S (2010) Chemical fixation of CO\(_2\): efficient synthesis of quinazoline-2,4(1H,3H)-diones catalyzed by guanidines under solvent-free conditions. Tetrahedron 66:4063–4067. doi:10.1016/j.tet.2010.04.011
Willis MC, Snell RH, Fletcher AJ, Woodward RL (2006) Tandem palladium-catalyzed urea arylation-intramolecular ester amidation? Regioselective synthesis of 3-alkylated 2,4-quinazolinediones. Org Lett 8:5089–5091. doi:10.1021/ol062009x
Beylin V, Boyles DC, Curran TT, Macikenas D, Parlett Vrieze D (2007) The preparation of two, preclinical amino-quinazolinediones as antibacterial agents. Org Process Res Dev 11:441–449. doi:10.1021/op7000639
Davidson JS (1984) The preparation of 5-(2-aminophenyl)-1,3,4-oxadiazole-2(3H)-one and its rearrangement to 3-amino-2,4(1H,3H)-quinazolinedione. Monatsh Chem Chem Mon 115:565–571. doi:10.1007/bf00799164
Ryu C-K, Shin K-H, Seo J-H, Kim H-J (2002) 6-Arylamino-5,8-quinazolinediones as potent inhibitors of endothelium-dependent vasorelaxation. Eur J Med Chem 37:77–82. doi:10.1016/s0223-5234(01)01290-9
Havera HJ (1979) Derivatives of 1,3-disubstituted 2,4(1H,3H)-quinazolinediones as possible peripheral vasodilators or antihypertensive agents. J Med Chem 22:1548–1550. doi:10.1021/jm00198a024
Usifoh CO, Scriba GKE (2000) Synthesis and anticonvulsant activity of acetylenic quinazolinone derivatives. Arch Pharm 333:261–266. doi:10.1002/1521-4184(20008)333:8<261:aid-ardp261>3.0.co;2-o
Lowe JA, Archer RL, Chapin DS, Cheng JB, Helweg D, Johnson JL, Koe BK, Lebel LA, Moore PF (1991) Structure–activity relationship of quinazolinedione inhibitors of calcium-independent phosphodiesterase. J Med Chem 34:624–628. doi:10.1021/jm00106a024
Fujimori H, Cobb DP (1965) Central nervous system depressant activity of Ma1337, 3-(3-(4-M-chlorophenyl-1-piperazyl)propyl)-2,4(1h,3h)quinazolinedione hydrochloride. J Pharmacol Exp Ther 148:151–157
Tran TP, Ellsworth EL, Stier MA, Domagala JM, Hollis Showalter HD, Gracheck SJ, Shapiro MA, Joannides TE, Singh R (2004) Synthesis and structural–activity relationships of 3-hydroxyquinazoline-2,4-dione antibacterial agents. Bioorg Med Chem Lett 14:4405–4409. doi:10.1016/j.bmcl.2004.06.063
Hassan MA, Younes AMM, Taha MM, Abdel-Monsef A (2012) Synthesis and reactions of 3-(2-chloromethyl-carbonylamino)tetrachloroquinazolin-2,4-dione. Org Chem Int 2012:1–4. doi:10.1155/2012/284947
Ryu CK, Shim JY, Yi YJ, Choi IH, Chae MJ, Han JY, Jung OJ (2004) Synthesis and antifungal activity of 5,8-quinazolinedione derivatives modified at positions 6 and 7. Arch Pharmacal Res 27:990–996. doi:10.1007/BF02975419
Elansary AK, Kadry HH, Ahmed EM, Sonousi ASM (2011) Design, synthesis, and biological activity of certain quinazolinedione derivatives as potent phosphodiestrase4 inhibitors. Med Chem Res 21:3557–3567. doi:10.1007/s00044-011-9892-x
Kirincich SJ, Xiang J, Green N, Tam S, Yang HY, Shim J, Shen MWH, Clark JD, McKew JC (2009) Benzhydrylquinazolinediones: novel cytosolic phospholipase A2\(\alpha \) inhibitors with improved physicochemical properties. Bioorg Med Chem 17:4383–4405. doi:10.1016/j.bmc.2009.05.027
Lansdon EB, Liu Q, Leavitt SA, Balakrishnan M, Perry JK, Lancaster-Moyer C, Kutty N, Liu X, Squires NH, Watkins WJ, Kirschberg TA (2011) Structural and binding analysis of pyrimidinol carboxylic acid and N-hydroxy quinazolinedione HIV-1 RNase H inhibitors. Antimicrob Agents Chemother 55:2905–2915. doi:10.1128/aac.01594-10
Malik M, Marks KR, Mustaev A, Zhao X, Chavda K, Kerns RJ, Drlica K (2011) Fluoroquinolone and quinazolinedione activities against wild-type and gyrase mutant strains of Mycobacterium smegmatis. Antimicrob Agents Chemother 55:2335–2343. doi:10.1128/aac.00033-11
Kakuta H, Tanatani A, Nagasawa K, Hashimoto Y (2003) Specific nonpeptide inhibitors of puromycin-sensitive aminopeptidase with a 2,4(1H,3H)-quinazolinedione skeleton. Chem Pharm Bull 51:1273–1282. doi:10.1248/cpb.51.1273
Ochoa-Diaz YO, Lopez-Moreno CY, Rendon-Maldonado JG, Lopez-Moreno HS (2012) Molecular diagnosis of Leishmania mexicana in a Cutaneous Leishmaniasis case in Sinaloa, Mexico. Vector-Borne Zoonotic Dis 12:78–80. doi:10.1089/vbz.2011.0688
Arguello-Garcia R, Cruz-Soto M, Romero-Montoya L, Ortega-Pierres G (2009) In vitro resistance to 5-nitroimidazoles and benzimidazoles in Giardia duodenalis: variability and variation in gene expression. Infect Genet Evol 9:1057–1064. doi:10.1016/j.meegid.2009.05.015
Navarrete-Vazquez G, Chavez-Silva F, Argotte-Ramos R, Rodriguez-Gutierrez Mdel C, Chan-Bacab MJ, Cedillo-Rivera R, Moo-Puc R, Hernandez-Nunez E (2011) Synthesis of benzologues of Nitazoxanide and Tizoxanide: a comparative study of their in vitro broad-spectrum antiprotozoal activity. Bioorg Med Chem Lett 21:3168–3171. doi:10.1016/j.bmcl.2011.02.100
Minodier P, Parola P (2007) Cutaneous leishmaniasis treatment. Travel Med Infect Dis 5:150–158. doi:10.1016/j.tmaid.2006.09.004
Sindermann H, Engel J (2006) Development of miltefosine as an oral treatment for leishmaniasis. Trans R Soc Trop Med Hyg 100:S17–S20. doi:10.1016/j.trstmh.2006.02.010
Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, Junge K, Bryceson A, Berman J (2002) Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med 347:1739–1746. doi:10.1056/NEJMoa021556
Paris C, Loiseau PM, Bories C, Breard J (2004) Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother 48:852–859. doi:10.1128/aac.48.3.852-859.2004
Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Diaz A, Luz M, Gutierrez P, Arboleda M, Berman JD, Junge K, Engel J, Sindermann H (2004) Miltefosine for new world Cutaneous Leishmaniasis. Clin Infect Dis 38:1266–1272. doi:10.1086/383321
Torres-Gomez H, Hernandez-Nunez E, Leon-Rivera I, Guerrero-Alvarez J, Cedillo-Rivera R, Moo-Puc R, Argotte-Ramos R, Rodriguez-Gutierrez Mdel C, Chan-Bacab MJ, Navarrete-Vazquez G (2008) Design, synthesis and in vitro antiprotozoal activity of benzimidazole-pentamidine hybrids. Bioorg Med Chem Lett 18:3147–3151. doi:10.1016/j.bmcl.2008.05.009
Bolivar P, Cruz-Paredes C, Hernández LR, Juárez ZN, Sánchez-Arreola E, Av-Gay Y, Bach H (2011) Antimicrobial, anti-inflammatory, antiparasitic, and cytotoxic activities of Galium mexicanum. J Ethnopharmacol 137:141–147. doi:10.1016/j.jep.2011.04.069
Martin-Quintal Z, Moo-Puc R, Gonzalez-Salazar F, Chan-Bacab MJ, Torres-Tapia LW, Peraza-Sanchez SR (2009) In vitro activity of Tridax procumbens against promastigotes of Leishmania mexicana. J Ethnopharmacol 122:463–467. doi:10.1016/j.jep.2009.01.037
Sarmiento-Sánchez JI, Montes-Avila J, Ochoa-Terán A, Delgado-Vargas F, Wilson-Corral V, Díaz-Camacho SP, García-Páez F, Bastidas-Bastidas P (2014) Synthesis of 1\(H\)-benzoxazine-2,4-diones from heterocyclic anhydrides: evaluation of antioxidant and antimicrobial activities. Quim Nova 37:1297–1301. doi:10.5935/0100-4042.20140201
Sarmiento-Sánchez JI, Ochoa-Terán A, Rivero IA (2014) Synthesis and antioxidant evaluation of enantiomerically pure bis-(1,2,3-triazolylmethyl)amino esters from modified\(\alpha \)-amino acids. Sci World J 2014:1–7. doi:10.1155/2014/264762
Washburne SS, Peterson WR Jr, Berman DA (1972) Reaction of trimethylsilyl azide with anhydrides and imides. A new uracil synthesis via nitrogen insertion. J Org Chem 37:1738–1742. doi:10.1021/jo00976a015
Nakagawa A, Uno S, Makishima M, Miyachi H, Hashimoto Y (2008) Progesterone receptor antagonists with a 3-phenylquinazoline-2,4-dione/2-phenylisoquinoline-1,3-dione skeleton. Bioorg Med Chem 16:7046–7054. doi:10.1016/j.bmc.2008.05.016
Papadopoulos EP, Torres CD (1982) Convenient preparation ofN-substituted 2-amino-4H-3, l-benzoxazin-4-ones and 3-substituted 2,4(1H,3H)-quinazolinediones. J Heterocycl Chem 19:269–272. doi:10.1002/jhet.5570190209
Steiger W, Kappe T, Ziegler E (1969) Synthesen von Heterocyclen, 123. Mitt.: Über Reaktionen des Isatosäureanhydrids mit Anilen. Monatsh Chem 100:146–149. doi:10.1007/bf00938250
Garin J, Melendez E, Merchán FL, Tejero T, Villarroya E (1983) Synthesis of 3-aryl-2,4-dioxo-1,2,3,4-tetrahydroquinazolines and 2-arylamino-4-oxo-4H-3,1-benzoxazines from methyl N-aryldithiocarbamates. Synthesis 1983:406–408. doi:10.1055/s-1983-30357
Horiie S, Murahashi S (1960) Studies on the high pressure reaction of carbon monoxide. III. Reaction between azocompounds and carbon monoxide. Bull Chem Soc Jpn 33:88–94. doi:10.1246/bcsj.33.88
Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J, Lacroix G, Hoet P (2009) Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part Fibre Toxicol 6:14. doi:10.1186/1743-8977-6-14
Schoen J (1995) The condensation of \(N, N^{\prime }\)-diaryl derivatives of thiourea with cyclohexanone. Synthesis of new compounds of the type 1,3-diaryl-2,4-dithioxo.ovrddot.octahydroquinazoline. Rocz Chem 29:549–566
Khademvatan S, Gharavi MJ, Akhlaghi L, Samadikuchaksaraei A, Oormazdi H, Mousavizadeh K, Hadighi R, Saki J (2009) Induction of apoptosis by miltefosine in iranian strain of Leishmania infantum promastigotes. Iran J Parasitol 4:23–31
Saibu GM, Katerere DR, Rees DJG, Meyer M (2015) In vitro cytotoxic and pro-apoptotic effects of water extracts of Tulbaghia violacea leaves and bulbs. J Ethnopharmacol 164:203–209. doi:10.1016/j.jep.2015.01.040
Lizarazo-Jaimes EH, Reis PG, Bezerra FM, Rodrigues BL, Monte-Neto RL, Melo MN, Frézard F, Demicheli C (2014) Complexes of different nitrogen donor heterocyclic ligands with SbCl\(_{3}\) and PhSbCl\(_{2}\) as potential antileishmanial agents against Sb\(^{III}\)-sensitive and -resistant parasites. J Inorg Biochem 132:30–36. doi:10.1016/j.jinorgbio.2013.12.001
Savoia D, Allice T, Tovo PA (2005) Antileishmanial activity of HIV protease inhibitors. Int J Antimicrob Agents 26:92–94. doi:10.1016/j.ijantimicag.2005.04.003
Acknowledgments
We gratefully acknowledge the support for this project by Consejo Nacional de Ciencia y Tecnología (SEP-CONACyT, GRANT No CB-2012-178266-Q) and Secretaría de Educación Pública (Nuevo PTC UAS-061, PROMEP/103.5/12/3360).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Juan I. Sarmiento-Sánchez and Héctor S. López-Moreno have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11030_2016_9693_MOESM1_ESM.docx
Supplementary data Available at http:// in the form of a PDF file, with free access: the 1H-NMR, 13C-NMR, and HRMS-ESI spectra for novel compounds (6c, 6e, and 6g). Graphs of percentage viabilities (promastigotes and macrophage cell) versus logarithmic concentrations are shown.
Rights and permissions
About this article
Cite this article
Enciso, E., Sarmiento-Sánchez, J.I., López-Moreno, H.S. et al. Synthesis of new quinazolin-2,4-diones as anti-Leishmania mexicana agents. Mol Divers 20, 821–828 (2016). https://doi.org/10.1007/s11030-016-9693-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9693-8