Abstract
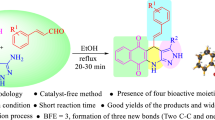
A series of pyrano-fused benzophenazines were synthesized using a bifunctional thiourea-based organocatalyst from the one-pot four-component reaction of 2-hydroxy-1,4-naphthoquinone, benzene-1,2-diamines, malononitrile or its derivatives and isatins or aromatic aldehydes in aqueous medium. Metal-free reaction condition, water as solvent, high bond forming efficiency (five new bonds formed in one step), good yields and easy purification process are the notable features of this methodology.
Graphical Abstract






Similar content being viewed by others
References
MacMillan DWC (2008) The advent and development of organocatalysis. Nature 455:304–308. doi:10.1038/nature07367
Dondoni A, Massi A (2008) Asymmetric organocatalysis: from infancy to adolescence organocatalysis. Angew Chem Int Ed 47:4638–4660. doi:10.1002/anie.200704684
Yu XH, Wang W (2008) Hydrogen-bond-mediated asymmetric catalysis. Asian J Chem 3:516–532. doi:10.1002/asia.200700415
Dalko PI, Moisan L (2004) In the golden age of organocatalysis. Angew Chem Int Ed 43:5138–5175. doi:10.1002/anie.200400650
Bertelsen S, Jorgensen KA (2009) Organocatalysis-after the gold rush. Chem Soc Rev 38:2178–2189. doi:10.1039/b903816g
List B (2007) Introduction: Organocatalysis. Chem Rev 107:5413–5415. doi:10.1021/cr078412e
Schreiner PR (2003) Metal-free organocatalysis through explicit hydrogen bonding interactions. Chem Soc Rev 32:289–296. doi:10.1039/B107298F
Doyle AG, Jacobsen EN (2007) Small-molecule H-bond donors in asymmetric catalysis. Chem Rev 107:5713–5743. doi:10.1021/cr068373r
Sigman MS, Jacobsen EN (1998) Schiff base catalysts for the asymmetric strecker reaction identified and optimized from parallel synthetic libraries. J Am Chem Soc 120:4901–4902. doi:10.1021/ja980139y
Schreiner PR, Wittkopp A (2002) H-bonding additives act like lewis acid catalysts. Org Lett 4:217–220. doi:10.1021/ol017117s
Okino T, Hoashi Y, Takemoto Y (2003) Enantioselective michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts. J Am Chem Soc 125:12672–12673. doi:10.1021/ja036972z
Takemoto Y (2005) Recognition and activation by ureas and thioureas: stereoselective reactions using ureas and thioureas as hydrogen-bonding donors. Org Biomol Chem 3:4299–4306. doi:10.1039/B511216H
Serdyuk OV, Heckel CM, Tsogoeva SB (2013) Bifunctional primary amine-thioureas in asymmetric organocatalysis. Org Biomol Chem 11:7051–7071. doi:10.1039/C3OB41403E
Fang X, Wang C-J (2015) Recent advances in asymmetric organocatalysis mediated by bifunctional amine-thioureas bearing multiple hydrogen-bonding donors. Chem Commun 51:1185–1197. doi:10.1039/C4CC07909D
Bugaut X, Constantieux T, Coquerel Y, Rodriguez J (2014) In: Zhu J,Wang Q, Wang M-X (eds) Multicomponent reactions in organic synthesis. Chap 5. Wiley, Weinheim, pp 109–158
Choudhury LH, Parvin T (2011) Recent advances in the chemistry of imine-based multicomponent reactions (MCRs). Tetrahedron 67:8213–8228. doi:10.1016/j.tet.2011.07.020
Rotstein BH, Zaretsky S, Rai V, Yudin AK (2014) Small heterocycles in multicomponent reactions. Chem Rev 114:8323–8359. doi:10.1021/cr400615v
Nair V, Rajesh V, Vinod A, Bindu US, Streekenth AR, Mathen JS, Balagopal L (2003) Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes. Acc Chem Res 36:899–907. doi:10.1021/ar020258p
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. doi:10.1021/cr0505728
Das D, Banerjee R, Mitra A (2014) Bioactive and pharmacologically important pyrano[2,3-c]pyrazoles. J Chem Pharmaceut Res 6:108–116
Malladi S, Isloora AM, Peethambar SK, Ganesh BM (2012) Palusa, Goud SK. Der Pharma Chem 4:43–52
Laursen JB, Nielsen J (2004) Phenazine natural products: Biosynthesis, synthetic analogues, and biological activity. J Chem Rev 104:1663–1686. doi:10.1021/cr020473
Hafez HN, Hegab MI, Ahmed-Farag IS, El-Gazzar ABA (2008) A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-\(9^\prime \),2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg Med Chem Lett 18:4538–4543. doi:10.1016/j.bmcl.2008.07.042
Mavrodi DV, Blankenfeldt W, Thomashow LS (2006) Phenazine compounds in fluorescent pseudomonas spp. biosynthesis and regulation. Annu Rev Phytopathol 44:417–445. doi:10.1146/annurev.phyto.44.013106.145710
Gamage SA, Spicer JA, Rewcastle GW, Milton J, Sohal S, Dangerfield W, Mistry P, Vicker N, Charlton PA, Denny WA (2002) Structure-activity relationships for pyrido-, imidazo-, pyrazolo-, pyrazino-, and pyrrolophenazinecarboxamides as topoisomerase-targeted anticancer agents. J Med Chem 45:740–743. doi:10.1021/jm010330
Tangmouo JG, Meli AL, Komguem J, Kuete V, Ngounou FN, Lontsi D, Beng VP, Choudhary MI, Sondengam BL (2006) Crassiflorone, a new naphthoquinone from Diospyros crassiflora (Hien). Tetrahedron Lett 47:3067–3070. doi:10.1016/j.tetlet.2006.03.006
Kraus GA, Kim IA (2003) A direct synthesis of \(o\)-methyl claussequinone. J Org Chem 68:4517–4518. doi:10.1021/jo030026j
Vicker N, Burgess L, Chuckowree IS, Dodd R, Folkes AJ, Hardick DJ, Hancox TC, Dangerfield W, Liddle C, Mistry P, Stewart AJ, Denny WA (2002) Novel angular benzophenazines: dual topoisomerase I and topoisomerase II inhibitors as potential anticancer agents. J Med Chem 45:721–739. doi:10.1021/jm010329a
Shahia M, Foroughifar N, Mobinikhaledi A (2015) Synthesis and antimicrobial activity of some tetrahydro quinolonediones and pyrano[2,3-d]pyrimidine derivatives. Iran J Pharm Res 14:757–763
Dar AM, uzzaman Shams (2015) Pathways for the synthesis of pyrimidine and pyran based hetrocyclic derivatives: a concise review. Eur Chem Bull 4:249–259. doi:10.17628/ECB.2015.4.249
de Andrade-Neto VF, Goulart MOF, da Silva Filho JF, da Silva MJ, do Pinto M CFR, Pinto AV, Zalis MG, Carvalho LH, Krettli AU (2004) Antimalarial activity of phenazines from lapachol, beta-lapachone and its derivatives against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorg Med Chem Lett 14:1145–1149. doi:10.1016/j.bmcl.2003.12.069
Feron O, Riant O, Kiss R, Leclercq J, Chataigne G, Vandelaer N, Lamy C (2013) Novel phenazine derivatives and their use. US Patent 20130289030 A1, 31 Oct 2013
Jardim GAM, Cruz EHG, Valença WO, Resende JM, Rodrigues BL, Ramos DF, Oliveira RN, Silva PEA, da Silva Júnior EN (2015) On the search for potential antimycobacterial drugs: synthesis of naphthoquinoidal, phenazinic and 1,2,3-triazolic compounds and evaluation against mycobacterium tuberculosis. J Braz Chem Soc 26:1013–1027. doi:10.5935/0103-5053.20150067
Hasaninejad A, Firoozi S (2013) One-pot, sequential four-component synthesis of benzo[c]pyrano[3,2-a]phena-zine, bis-benzo[c]pyrano[3,2-a]phenazine and oxospiro benzo[c]pyrano[3,2-a]phenazine derivatives using 1,4-diazabicyclo[2.2.2]octane (DABCO) as an efficient and reusable solid base catalyst. Mol Divers 17:499–513. doi:10.1007/s11030-013-9446-x
Wang SL, Wu F-Y, Cheng C, Zhang G, Liu Y-P, Jiang B, Shi F, Ju S-J (2011) Multicomponent synthesis of poly-substituted benzo[\(a\)]pyrano[2,3-\(c\)]phenazine derivatives under microwave heating. ACS Comb Sci 13:135–139. doi:10.1021/co1000376
Mahdavinia GH, Mirzazadeh M, Notash B (2013) A rapid and simple diversity-oriented synthesis of novel 3-amino-\(2^\prime \)-oxospiro [benzo[\(c\)]pyrano[3,2-\(a\)]phenazine-1,\(3^\prime \)-indoline]-2-carbonitrile/carboxylate derivatives via a one-pot, four-component domino reaction. Tetrahedron Lett 54:3487–3492. doi:10.1016/j.tetlet.2013.04.082
Hasaninejad A, Firoozi S, Mandegani F (2013) An efficient synthesis of novel spiro[benzo[\(c\)]pyrano[3,2-\(a\)]phenazines] via domino multi-component reactions using l-proline as a bifunctional organocatalyst. Tetrahedron Lett 54:2791–2794. doi:10.1016/j.tetlet.2013.03.073
Elah Abadi AY, Maghsoodlou M-T, Heydari R, Mohebat R (2015) PTSA-catalyzed four-component domino reactions for the one-pot synthesis of functionalized 11H-benzo[a]benzo[6,7]chromeno[2,3-c]phenazine-11,16(17H)-diones in PEG. Res Chem Intermed. doi:10.1007/s11164-015-2083-5
Bharti R, Parvin T (2015) Diversity oriented synthesis of tri-substituted methane containing aminouracil and hydroxynaphthoquinone /hydroxycoumarin moiety using organocatalysed multicomponent reactions in aqueous medium. RSC Adv 5:66833–66839. doi:10.1039/c5ra13093j
Bharti R, Parvin T (2015) Molecular Diversity from the L-proline catalyzed, three-component reactions of 4-hydroxycoumarin, aldehyde, and 3-aminopyrazole or 1,3-dimethyl-6-aminouracil. Synth Commun 45:1442–1450. doi:10.1002/chin.201537164
Bharti R, Parvin T (2015) One-pot synthesis of highly functionalized tetrahydropyridines: a camphoresulfonic acid catalyzed multicomponent reaction. J Heterocycl Chem 52:1806–1811. doi:10.1002/jhet.2268
Karamthulla S, Pal S, Parvin T, Choudhury LH (2014) L-proline catalyzed multicomponent reactions: facile access to 2H-benzo[g]pyrazolo[3,4-b]quinoline-5,10(4H,11H)-dione derivatives. RSC Adv 4:15319–15324. doi:10.1039/c4ra00876f
Pal S, Parvin T, Choudhury LH (2012) \(\text{ VCl }_{3}\) catalyzed imine-based multicomponent reactions for the facile access of functionalized tetrahydropyridines and \(\upbeta \)-amino carbonyls. Mol Divers 16:129–143. doi:10.1007/s11030-011-9339-9
Khan AT, Parvin T, Choudhury LH (2008) Effects of substituent in \(\beta \)-position of 1, 3-dicarbonyl compounds in bromodimethylsulfonium bromide catalyzed multicomponent reactions: a facile access to functionalized piperidines. J Org Chem 73:8398–8402. doi:10.1021/jo8014962
Acknowledgments
We are grateful to NIT Patna and the Department of Science and Technology, India for the financial support with Sanction No. SR/FT/CS-008/2010. The authors are grateful to IIT Patna and SAIF-Panjab University for providing the analytical facilities for characterization of products.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bharti, R., Parvin, T. Multicomponent synthesis of diverse pyrano-fused benzophenazines using bifunctional thiourea-based organocatalyst in aqueous medium. Mol Divers 20, 867–876 (2016). https://doi.org/10.1007/s11030-016-9681-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9681-z