Abstract
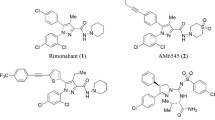
Peripherally acting cannabinoid 1 (CB1) receptor antagonists are considered as potential therapeutics for the treatment of obesity with desired efficacy and reduced central nervous system side effects. A dataset of 72 compounds containing the 1,5-diaryl pyrazole basic skeleton having peripheral CB1 receptor antagonistic activity was utilized for three-dimensional quantitative structure–activity relationship studies. Compounds of the series exhibited high variations in the biological activity and chemical structures. Different types of molecular alignments, such as atom-based, data-based, centroid-based and centroid/atom-based were utilized to develop the best CoMFA model. The best CoMFA model was obtained with a database alignment and the same alignment was further used for the development of a CoMSIA model. The best developed CoMFA model had \(r^{2}_\mathrm{cv} = 0.552\) with six components, \(r^{2}_\mathrm{ncv} = 0.973,\,\mathrm{standard\,error\,of\,estimate} = 0.162,\,F{\text {-value}} = 281.239,\) while the best developed CoMSIA model had \(r^{2}_\mathrm{cv} = 0.571\) with six components, \(r^{2}_\mathrm{ncv} = 0.960,\, \mathrm{standard} \mathrm{error\,of\,estimate} = 0.196\) and \(F{\text {-value}} = 188.701.\) The predictive \(r^{2}\) values of these two models showed test set predictions of 0.528 and 0.679 for the best CoMFA and CoMSIA models, respectively. Based on a higher \(r^{2}_\mathrm{pred}\) value, the CoMSIA model was found to be the best one. The prediction accuracy and reliability of the best developed CoMSIA model have been validated using well-established methods. Using the inputs from the best CoMSIA contour maps, several novel highly selective peripherally acting CB1 receptor antagonists have been designed and reported herein.










Similar content being viewed by others
References
http://www.mexicobariatriccenter.com/obesity-statistics-2013-usa-world. Accessed 13 Oct 2014
Swinburn BA, Caterson I, Seidell JC, James WPT (2004) Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr 7:123–146. doi:10.1079/PHN2003585
Halford JC (2001) Pharmacology of appetite suppression: implication for the treatment of obesity. Curr Drug Targets 2:353–370. doi:10.2174/1389450013348209
Kirilly E, Gonda X, Bagdy G (2012) CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol 205:1–20. doi:10.1111/j.1748-1716.2011.02402.x
http://www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed 13 Oct 2014
Rodgers RJ, Tschop MH, Wilding JPH (2012) Anti-obesity drugs: past, present and future. Dis Model Mech 5:621–626. doi:10.1242/dmm.009621
Powell AG, Apovian CM, Aronne LJ (2011) New drug targets for the treatment of obesity. Clin Pharmacol Ther 90:40–51. doi:10.1038/clpt.2011.82
Di Marzo V, Despres JP (2009) CB1 antagonists for obesity—what lessons have we learned from rimonabant? Nat Rev Endocrinol 5:633–638. doi:10.1038/nrendo.2009.197
Pertwee RG (1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74:129–180. doi:10.1016/S0163-7258(97)82001-3
Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F (2010) The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav 95:375–382. doi:10.1016/j.pbb.2010.03.012
Vettor R, Pagano C (2009) The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract Res Clin Endocrinol Metab 23:51–63. doi:10.1016/j.beem.2008.10.002
Yen-Ku W, Ching-Fang Y, Tai WL, Ming-Shiu H (2011) A new perspective of cannabinoid 1 receptor antagonists: approaches toward peripheral CB1R blockers without crossing the blood–brain barrier. Curr Top Med Chem 11:1421–1429. doi:10.2174/156802611795860997
Kennett GA, Clifton PG (2010) New approaches to the pharmacological treatment of obesity: can they break through the efficacy barrier? Pharmacol Biochem Behav 97:63–83. doi:10.1016/j.pbb.2010.07.020
Leite CE, Mocelin CA, Petersen GO, Leal MB, Thiesen FV (2009) Rimonabant: an antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol Rep 61:217–224. doi:10.1016/S1734-1140(09)70025-8
Sharma MK, Murumkar PR, Kanhed AM, Giridhar R, Yadav MR (2014) Prospective therapeutic agents for obesity: molecular modification approaches of centrally and peripherally acting selective cannabinoid 1 receptor antagonists. Eur J Med Chem 79:298–339. doi:10.1016/j.ejmech.2014.04.011
Hung MS, Chang CP, Li TC, Yeh TK, Song JS, Lin Y, Wu CH, Kuo PC, Amancha PK, Wong YC, Hsiao WC, Chao YS, Shia KS (2010) Discovery of 1-(2,4-dichlorophenyl)-4-ethyl-5-(5-(2-(4-(trifluoromethyl)phenyl)ethynyl)thiophen-2-yl)-N-(piperidin-1-yl)-1Hpyrazole-3-carboxamide as a potential peripheral cannabinoid-1 receptor inverse agonist. Chem Med Chem 5:1439–1443. doi:10.1002/cmdc.201000246
Klumpers LE, Fridberg M, de Kam ML, Little PB, Jensen NO, Kleinloog HD, Elling CE, van Gerven JM (2013) Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br J Clin Pharmacol 76:846–857. doi:10.1111/bcp.12141
Quarta C, Mazza R, Obici S, Pasquali R, Pagotto U (2011) Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med 17:518–526. doi:10.1016/j.molmed.2011.05.002
Douguet D, Munier-Lehmann H, Labesse G, Pochet S (2005) LEA3D: a computer-aided ligand design for structure-based drug design. J Med Chem 48:2457–2468. doi:10.1021/jm0492296
Wang P, Cai J, Chen J, Li L, Sun C, Xue B, Ji M (2014) 3D-QSAR and docking studies of piperidine carboxamide derivatives as ALK inhibitors. Med Chem Res 23:2576–2583. doi:10.1007/s00044-013-0853-4
Murumkar PR, Giridhar R, Yadav MR (2008) 3D-quantitative structure–activity relationship studies on benzothiadiazepine hydroxamates as inhibitors of tumor necrosis factor-\(\alpha \) converting enzyme. Chem Biol Drug Des 71:363–373. doi:10.1111/j.1747-0285.2008.00639.x
Murumkar PR, DasGupta S, Zambre VP, Giridhar R, Yadav MR (2009) Development of predictive 3D-QSAR CoMFA and CoMSIA models for \(\beta \)-aminohydroxamic acid-derived tumor necrosis factor-\(\alpha \) converting enzyme inhibitors. Chem Biol Drug Des 73:97–107. doi:10.1111/j.1747-0285.2008.00737.x
Murumkar PR, Zambre VP, Yadav MR (2010) Development of predictive pharmacophore model for in silico screening, and 3D QSAR CoMFA and CoMSIA studies for lead optimization, for designing of potent tumor necrosis factor alpha converting enzyme inhibitors. J Comput Aided Mol Des 24:143–156. doi:10.1007/s10822-010-9322-z
Murumkar PR, Le L, Truong TN, Yadav MR (2011) Determination of structural requirements of influenza neuraminidase type A inhibitors and binding interaction analysis with the active site of A/H1N1 by 3D-QSAR CoMFA and CoMSIA modeling. Med Chem Commun 2:710–719. doi:10.1039/c1md00050k
Murumkar PR, Sharma MK, Shinde AC, Bothara KG (2013) Three-dimensional quantitative structure–activity relationship CoMFA/CoMSIA on pyrrolidine-based tartrate diamides as TACE inhibitors. Med Chem Res 22:4192–4201. doi:10.1007/s00044-012-0409-z
Murumkar PR, Sharma MK, Giridhar R, Yadav MR (2014) Virtual screening-based identification of lead molecules as selective TACE inhibitors. Med Chem Res 24:226–244. doi:10.1007/s00044-014-1097-7
Zambre VP, Murumkar PR, Giridhar R, Yadav MR (2009) Structural investigations of acridine derivatives by CoMFA and CoMSIA reveal novel insight into their structures toward DNA G-Quadruplex mediated telomerase inhibition and Offer a highly predictive 3D-model for substituted Acridines. J Chem Inf Model 49:1298–1311. doi:10.1021/ci900036w
Zambre VP, Murumkar PR, Giridhar R, Yadav MR (2010) Development of highly predictive 3D-QSAR CoMSIA models for anthraquinone and acridone derivatives as telomerase inhibitors targeting G-quadruplex DNA telomere. J Mol Graph Model 29:229–239. doi:10.1016/j.jmgm.2010.07.003
Zambre VP, Giridhar R, Yadav MR (2013) Pharmacophore modeling and 3D-QSAR (CoMSIA) studies for structural requirements of some triazine derivatives as G-quadruplex binders for telomerase inhibition. Med Chem Res 22:4685–4699. doi:10.1007/s00044-012-0447-6
Puntambekar DS, Giridhar R, Yadav MR (2006) 3D-QSAR CoMFA/CoMSIA studies on 5-aryl-2,2-dialkyl-4-phenyl-3(2H)-furanone derivatives, as selective COX-2 inhibitors. Acta Pharm 56:157–174
Puntambekar DS, Giridhar R, Yadav MR (2006) 3D-QSAR studies of farnesyltransferase inhibitors: a comparative molecular field analysis approach. Bioorg Med Chem Lett 16:1821–1827. doi:10.1016/j.bmcl.2006.01.019
Puntambekar DS, Giridhar R, Yadav MR (2006) Understanding the antitumor activity of novel tricyclicpiperazinyl derivatives as farnesyltransferase inhibitors using CoMFA and CoMSIA. Eur J Med Chem 41:1279–1292. doi:10.1016/j.ejmech.2006.07.002
Puntambekar DS, Giridhar R, Yadav MR (2008) Insights into the structural requirements of farnesyltransferase inhibitors as potential anti-tumor agents based on 3D-QSAR CoMFA and CoMSIA models. Eur J Med Chem 43:142–154. doi:10.1016/j.ejmech.2007.02.003
Hernandez-Vazquez E, Mendez-Lucio O, Hernandez-Luis F (2013) Activity landscape analysis, CoMFA and CoMSIA studies of pyrazole CB1 antagonists. Med Chem Res 22:4133–4145. doi:10.1007/s00044-012-0418-y
Sasmal PK, Reddy DS, Talwar R, Venkatesham B, Balasubrahmanyam D, Kannan M, Srinivas P, Kumar KS, Devi BN, Jadhav VP, Khan SK, Mohan P, Chaudhury H, Bhuniya D, Iqbal J, Chakrabarti R (2011) Novel pyrazole-3-carboxamide derivatives as cannabinoid-1 (CB1) antagonists: journey from non-polar to polar amides. Bioorg Med Chem Lett 21:562–568. doi:10.1016/j.bmcl.2010.10.055
Sasmal PK, Talwar R, Swetha J, Balasubrahmanyam D, Venkatesham B, Rawoof KA, Devi BN, Jadhav VP, Khan SK, Mohan P, Reddy DS, Nyavanandi VK, Nanduri S, Kumar SK, Kannan M, Srinivas P, Nadipalli P, Chaudhury H, Sebastian VJ (2011) Structure–activity relationship studies of novel pyrazole and imidazole carboxamides as cannabinoid-1 (CB1) antagonists. Bioorg Med Chem Lett 21:4913–4918. doi:10.1016/j.bmcl.2011.06.017
SYBYL 7.0. Tripos, Inc., St. Louis
Adhikari N, Halder AK, Mondal C, Jha T (2013) Exploring structural requirements of aurone derivatives as antimalarials by validated DFT-based QSAR, HQSAR, and COMFA-COMSIA approach. Med Chem Res 22:6029–6045. doi:10.1007/s00044-013-0590-8
Cramer RD, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110:5959–5967. doi:10.1021/ja00226a005
Zha C, Brown GB, Brouillette WJ (2014) A highly predictive 3D-QSAR model for binding to the voltage-gated sodium channel: design of potent new ligands. Bioorg Med Chem 22:95–104. doi:10.1016/j.bmc.2013.11.049
Kliebe G, Abraham U, Mietzner T (1994) Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J Med Chem 37:4130–4146. doi:10.1021/jm00050a010
Cramer RD, Bunce JD, Patterson DE (1988) Crossvalidation, bootstrapping, and partial least squares compared with multiple regression in conventional QSAR studies. Quant Struct Act Relatsh 7:18–25. doi:10.1002/qsar.19880070105
Kamath S, Buolamwini JK (2003) Receptor-guided alignment-based comparative 3D-QSAR studies of benzylidene malonitrile tyrphostins as EGFR and HER-2 kinase inhibitors. J Med Chem 46:4657–4668. doi:10.1021/jm030065n
Araujo JQ, de Brito MA, Hoelz LVB, de Alencastro RB, Castro HC, Rodrigues CR, Albuquerque MG (2011) Receptor-dependent (RD) 3D-QSAR approach of a series of benzylpiperidine inhibitors of human acetylcholinesterase (HuAChE). Eur J Med Chem 46:39–51. doi:10.1016/j.ejmech.2010.10.009
Dunn WJ, Wold S, Edlund V, Helberg S (1984) Multivariate structure–activity relationship between data from a battery of biological tests and an ensemble of structure descriptors: the PLS method. Quant Struct Act Relatsh 3:131–137. doi:10.1002/qsar.19840030402
Hansch C, Verma RP (2009) Overcoming tumor drug resistance with C\(_{2}\)-modified 10-deacetyl-7-propionyl cephalomannines: a QSAR study. Mol Pharm 6:849–860. doi:10.1021/mp800138w
Verma RP, Hansch C (2008) Combating the threat of anthrax: a quantitative structure–activity relationship approach. Mol Pharm 5:745–759. doi:10.1021/mp8000149
Kristama R, Parmara V, Viswanadhana VN (2013) 3D-QSAR analysis of TRPV1 inhibitors reveals a pharmacophore applicable to diverse scaffolds and clinical candidates. J Mol Graph Model 45:157–172. doi:10.1016/j.jmgm.2013.08.014
Liu Y, Lu X, Xue T, Hu S, Zhang H (2014) Receptor and ligand-based 3D-QSAR study on a series of pyrazines/pyrrolidylquinazolines as inhibitors of PDE10A enzyme. Med Chem Res 23:775–789. doi:10.1007/s00044-013-0619-z
Roy PP, Paul S, Mitra I, Roy K (2009) On two novel parameters for validation of predictive QSAR models. Molecules 14:1660–1701. doi:10.3390/molecules14051660
Roy PP, Roy K (2008) On some aspects of variable selection for partial least squares regression models. QSAR Comb Sci 27:302–313. doi:10.1002/qsar.200710043
Golbraikh A, Tropsha A (2002) Beware of q2!. J Mol Graph Model 20:269–276. doi:10.1016/S1093-3263(01)00123-1
Melagraki G, Afantitis A (2013) Enalos KNIME nodes: exploring corrosion inhibition of steel in acidic medium. Chemom Intell Lab Syst 123:9–14. doi:10.1016/j.chemolab.2013.02.003
Zhang S, Golbraikh A, Oloff S, Kohn H, Tropsha A (2006) A novel automated lazy learning QSAR (ALL-QSAR) approach: method development, applications, and virtual screening of chemical databases using validated ALL-QSAR models. J Chem Inf Model 46:1984–1995. doi:10.1021/ci060132x
Melagraki G, Afantitis A (2014) Enalos InSilicoNano platform: an online decision support tool for the design and virtual screening of nanoparticles. RSC Adv 4:50713–50725. doi:10.1039/C4RA07756C
Dossou KS, Devkota KP, Kavanagh PV, Beutler JA, Egan JM, Moaddel R (2013) Development and preliminary validation of a plate-based CB1/CB2 receptor functional assay. Anal Biochem 437:138–143. doi:10.1016/j.ab.2013.02.025
Cooper M, Receveur JM, Bjurling E, Norregaard PK, Nielsen PA, Skold N, Hogberg T (2010) Exploring SAR features in diverse library of 4-cyanomethyl-pyrazole-3-carboxamides suitable for further elaborations as CB1 antagonists. Bioorg Med Chem Lett 20:26–30. doi:10.1016/j.bmcl.2009.11.047
Receveur J-M, Murray A, Linget J-M et al (2010) Conversion of 4-cyanomethyl-pyrazole-3-carboxamides into CB1 antagonists with lowered propensity to pass the blood–brain-barrier. Bioorg Med Chem Lett 20:453–457. doi:10.1016/j.bmcl.2009.12.003
Sharma MK, Murumkar PR, Barmade MA, Giridhar R, Yadav MR (2015) A comprehensive patents review on cannabinoid 1 receptor antagonists as antiobesity agents. Expert Opin Ther Pat. doi:10.1517/13543776.2105.1064898
Qikprop version 3.2. Schrödinger, LLC, New york (2009)
Acknowledgments
MKS is thankful to University Grants Commission (UGC), New Delhi for awarding Junior Research Fellowship (JRF) under the RFSMS-BSR Programme [No. F. 7-129/2007 (BSR)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in preparation of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, M.K., Murumkar, P.R., Giridhar, R. et al. Exploring structural requirements for peripherally acting 1,5-diaryl pyrazole-containing cannabinoid 1 receptor antagonists for the treatment of obesity. Mol Divers 19, 871–893 (2015). https://doi.org/10.1007/s11030-015-9611-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9611-5