Abstract
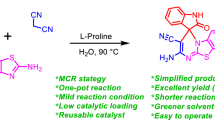
An efficient and diastereoselective synthetic procedure for highly functionalized tetrahydroacenaphtho[1,2-\(b\)]indolone derivatives was successfully developed by the three-component reaction of acenaphthequinone, enaminones, and barbituric acid in the presence of a catalytic amount of L-proline. This method has the advantages of convenient operation, excellent yields, mild reaction conditions, and environmental friendliness.


Similar content being viewed by others
References
Dömling A, Wang W, Wang K (2012) Chemistry and biology of multicomponent reaction. Chem Rev 112:3083–3135. doi:10.1021/cr100233r
Tietze LF, Brasche G, Gericke K (2006) Domino reactions in organic synthesis. Wiley-VCH, Weinheim
Tietze LF (1996) Domino reactions in organic synthesis. Chem Rev 96:115–136. doi:10.1021/cr950027e
Dömling A (2006) Recent develpoments in isocyanide based mnlticomponent reactions in applied chemistry. Chem Rev 106:17–89. doi:10.1021/cr0505728
Ramón DJ, Yus M (2005) Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew Chem Int Ed 44:1602–1634. doi:10.1002/anie.200460548
Dömling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168–3210. doi:10.1002/1521-3773(10000915)39:18<3168:AID-ANIE3168>3.0.CO;2-U
Li M, Li T, Zhao K, Wang M, Wen R (2013) Application of functionalized N,S-ketene acetals-microwave-assisted three-component domino reaction for rapid direct acess to imidazo[1,2-a]pyridines. Chin J Chem 31:1033–1038. doi:10.1002/cjoc.201300252
Liu Z, Zhang L, Sun J, Yang C (2014) Diastereoselective synthesis of functionalized tetrahydropyrimidin-2-thiones via \(\text{ ZnCl }_{2}\) promoted one-pot reactions. Chin J Chem 32:172–178. doi: 10.1002/cjoc.201300577
Gamage SA, Tepsiri N, Wilairat P, Wojcik SJ, Figgitt DP, Ralph RK, Denny WA (1994) Synthesis and in vitro evaluation of 9-anilino-3,6-diaminoacridines active against a multidrug—resistant strain of the malaria parasite plasmodium falciparum. J Med Chem 37:1486–1494. doi:10.1021/jm00036a014
Eren G, Unlu S, Nunez MT, Labeaga L, Ledo F, Entrena A, Lu EB, Costantino G, Sahin MF (2010) Synthesis, biological evaluation, and docking studies of novel heterocyclic diaryl compounds as selective COX-2 inhibitors. Bioorg Med Chem 18:6367–6376. doi:10.1016/j.bmc.2010.07.009
Michaudel Q, Thevenet D, Baran PS (2012) Intermolecular Ritter-type C–H amination of unactivated \(\text{ sp }^{3}\) carbons. J Am Chem Soc 134:2547–2550. doi: 10.1021/ja212020b
Iglesias A, Alvarez R, Lea AR, Muniz K (2012) Palladium-catalyzed intermolecular \(\text{ C }(\text{ sp }^{3})\)–H amidation. Angew Chem Int Ed 51:2225–2228. doi: 10.1002/anie.201108351
Fan R, Li W, Pu D, Zhang L (2009) Transition-metal-free intermolecular amination of \(\text{ sp }^{3}\) C–H bonds with sulfonamides. Org Lett 11:1425–1428. doi: 10.1021/ol90009of
Davies HML, Long MS (2005) Recent advances in catalytic intramolecular C–H aminations. Angew Chem Int Ed 44:3518–3520. doi:10.1002/anie.200500554
Davies HML (2006) Recent advances in catalytic enantioselective intermolecular C–H functionalization. Angew Chem Int Ed 45:6422–6425. doi:10.1022/anie.200601814
Fuchs JR, Funk RL (2005) Indol-2-one intermediates: mechanistic evidence and synthetic utility. Total syntheses of (\(\pm \))-Flustramines A and C. Org Lett 7:677–680. doi:10.1021/ol047532v
Chen WL, Cai YF, Fu X, Liu XH, Liu LL, Feng XM (2011) Enantioselective one-pot synthesis of 2-amino-4-(indol-3-yl)-4H-chromenes. Org Lett 13:4910–4913. doi:10.1021/ol2019949
Thirumurugan P, Perumal PT (2009) \(\text{ InCl }_{3}\) mediated one-pot synthesis of indol-3-yl pyridine and \(2,2^{\prime } \)-bipyridine derivatives through multi-component reaction. Tetrahedron 65:7620–7629. doi:10.1016/j.tet.2009.06.097
Shi F, Xing GJ, Zhu RY, Tan W, Tu SJ (2013) A catalytic asymmetric isatin-involved Povarov reaction: diastereo- and enantioselective construction of spiro[indolin-3,2\({\prime }\)-quinoline] scaffold. Org Lett 15:128–131. doi:10.1021/ol303154k
Li C, Guo F, Xu K, Zhang S, Hu Y, Zha Z, Wang Z (2014) Copper-catalyzed enantioselective Friedel–Crafts allylation of pyrrole with isatins. Org Lett 16:3192–3195. doi:10.1021/ol501086q
Zi Y, Cai ZJ, Wang SY, Ji SJ (2014) Synthesis of isatins by \(\text{ I }_{2}\)/TBHP mediated oxidation of indoles. Org Lett 16:3094–3097. doi: 10.1021/ol501203q
Hao WJ, Wang SY, Ji SJ (2013) Iodine-catalyzed cascade formal [3+3] cycloaddition reaction of indolyl alcohol derivatives with enaminones: construction of functionalized spirodihydrocarbolines. ACS Catal 3:2501–2504. doi:10.1021/cs400703u
Jiang B, Li QY, Zhang H, Tu SJ, Pindi S, Li G (2012) Efficient domino approaches to multifunctionalized fused pyrroles and dibenzo[b, e][1,4]diazepin-1-ones. Org Lett 14:700–703. doi:10.1021/ol203166c
Jiang B, Li QY, Tu SJ, Li G (2012) Three-component domino reactions for selective formation of indeno[1,2-b]indole derivatives. Org Lett 14:5210–5213. doi:10.1021/ol3023038
Fu LP, Shi QQ, Shi F, Jiang B, Tu SJ (2013) Three-component domino reactions for regioselective formation of bis-indole derivatives. ACS Comb Sci 15:135–140. doi:10.1021/co3001428
List B, Lerner RA, Barbas CF III (2000) Proline-catalyzed direct asymmetric Aldol reactions. J Am Chem Soc 122:2395–2396. doi:10.1021/ja994280y
List B (2004) Enamine catalyzed is a powerful strategy for the catalytic generation and use of carbanion equivalents. Acc Chem Res 37:548–557. doi:10.1021/ar0300571
Mukherjee S, Yang JW, Hoffmann S, List B (2007) Asymmetric enamine catalysis. Chem Rev 107:5471–5569. doi:10.1021/cr0684016
Ramachary DB, Chowdari NS, Barbas CF III (2003) Organocatalytic asymmetric Domino Knoevenagel/Diels–Alder reactions: a bioorganic approach to the diastereospecific and enantioselective construction of highly substituted spiro[5,5]undecane-1,5,9-triones. Angew Chem 115:4365–4369. doi:10.1002/ange.200351916
Notz W, Tanaka F, Barbas CF III (2004) Enamine-based organocatalysis with proline and diamines: the developments of direct catalytic asymmetric Aldol, Mannich, Michael, and Diels–Alder reactions. Acc Chem Res 37:580–591. doi:10.1021/ar0300468
Jiang H, Mai R, Cao H, Zhu Q, Liu X (2009) L-Proline-catalyzed synthesis of highly functionalized multisubstituted 1,4-dihydropyridines. Org Biomol Chem 7:4943–4953. doi:10.1039/B914659H
Rajesh SM, Bala BD, Perumal S, Menéndez JC (2011) L-Proline-catalyzed sequential four-component “on water” protocol for the synthesis of structurally complex heterocyclic ortho-quinones. Green Chem 13:3248–3254. doi:10.1039/C1GC15794A
Abdolmohammadi S, Balalaie S (2007) Novel and efficient catalysts for the one-pot synthesis of 3,4-dihydropyrano[c]chromane derivatives in aqueous media. Tetrahedron Lett 48:3299–3303. doi:10.1016/j.tetlet.2007.02.135
Kumar A, Maurya RA (2007) Synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction using organocatalysts. Tetrahedron 63:1946–1952. doi:10.1016/j.tet.2006.12.074
Shi CL, Shi DQ, Kim SH, Huang ZB, Ji SJ, Ji M (2008) A novel and efficient one-pot synthesis of furo[3’,4’:5,6]pyrido[2,3-\(c\)]pyrazole derivatives using organocatalysts. Tetrahedron 64:2425–2432. doi: 10.1016/j.tet.2007.12.053
Shi CL, Chen H, Li Y, Shi DQ, Ji M (2008) A three-component synthesis of N-substituted quinoline-3-carbonitrile derivatives catalyzed by L-proline. J Chem Res 32:534–537. doi:10.3184/030823408X347567
Shi CL, Shi DQ (2011) Green synthesis of chromen-2-one derivatives catalyzed by L-proline. J Chem Res 35:585–586. doi:10.3184/174751911X13173059031452
Wang HY, Li LL, Lin W, Huang ZB, Shi DQ (2013) Progress in application of L-proline in catalyzing the synthesis of heterocyclic compounds. Chin J Org Chem 33:1616–1627. doi:10.6023/cjoc201210033
Kaur P, Pindi S, Wever W, Rajale T, Li G (2010) Asymmetric catalytic Strecker reaction of \(N\)-phosphonyl imines with \(\text{ Et }_{2}\)AlCN using amino alcohols and BINOLs as catalyst. Chem Commun 46:4330–4332. doi: 10.1039/C0CC00287A
Kaur P, Pindi S, Wever W, Rajale T, Li G (2010) Asymmetric catalytic \(N\)-phosphonyl imine chemistry: the use of primary free amino acids and \(\text{ Et }_{2}\)AlCN for asymmetric Strecker reaction. J Org Chem 75:5244–5250. doi: 10.1021/jo100865q
Kaur P, Wever W, Pindi S, Milles R, Gu P, Shi M, Li G (2011) The GAP chemistry for chiral \(N\)-phosphonyl imine-based Strecker reaction. Green Chem 13:1288–1292. doi: 10.1039/C1GC15029D
Acknowledgments
This work was financially supported by the Natural Science Foundation of Jiangsu Province (No. BK20131160), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 11KJB150014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, JJ., Feng, X., Liu, XC. et al. An efficient three-component synthesis of highly functionalized tetrahydroacenaphtho[1,2-\(b\)]indolone derivatives catalyzed by L-proline. Mol Divers 18, 727–736 (2014). https://doi.org/10.1007/s11030-014-9544-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-014-9544-4