Abstract
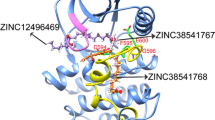
B-RAF is a member of the RAF protein kinase family involved in the regulation of cell growth, differentiation, and proliferation. It forms a part of conserved apoptosis signals through the RAS–RAF–MAPK pathway. V600EB-RAF protein has much potential for scientific research as therapeutic target due to its involvement in human melanoma cancer. In this work, a molecular modeling study was carried out for the first time with 3D-QSAR studies by following the docking protocol on three different data sets of V600EB-RAF inhibitors. Based on the co-crystallized compound (PDB ID: 1UWJ), a receptor-guided alignment method was utilized to derive reliable CoMFA and CoMSIA models. The selected CoMFA model gives the best statistical values (q 2 = 0.753, r 2 = 0.962). With the same alignment protocol, a statistically reliable CoMSIA model out of fourteen different combinations was also derived (q 2 = 0.807, r 2 = 0.961). The actual predictive powers of both models were rigorously validated with an external test set, which gave satisfactory predictive r 2 values for CoMFA and CoMSIA models, 0.89 and 0.88, respectively. In addition, y-randomization test was also performed to validate our 3D-QSAR models. Contour maps from CoMFA and CoMSIA models supported statistical results, revealed important structural features responsible for biological activity within the active site and explained the correlation between biological activity and receptor–ligand interactions. Based on the developed models few new structures were designed. The newly predicted structure (IIIa) showed higher inhibitory potency (pIC50 6.826) than that of the most active compound of the series.
Similar content being viewed by others
References
Wilhelm SM, Carter C, Tang LY, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099–7109. doi:10.1158/0008-5472.CAN-04-1443
Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK (2008) Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci 105: 3041–3046. doi:10.1073/pnas.0711741105
Takle AK, Bamford MJ, Davies S, Davis RP, Dean DK, Gaiba A, Irving EA, King FD, Naylor A, Parr CA (2008) The identification of potent, selective and CNS penetrant furan-based inhibitors of B-Raf kinase. Bioorg Med Chem Lett 18: 4373–4376. doi:10.1016/j.bmcl.2008.06.070
Garnett MJ, Marais R (2004) Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6: 313–319. doi:10.1016/j.ccr.2004.09.022
Mercer KE, Pritchard CA (2003) Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta 1653: 25–40. doi:10.1016/S0304-419X(03)00016-7
Chung JY, Chung HW, Cho SJ, Hah JM, Cho AE (2010) QM/MM based 3D QSAR models for potent B-Raf inhibitors. J Comput Aided Mol Des 24: 385–397. doi:10.1007/s10822-010-9337-5
Montagut C, Settleman J (2009) Targeting the RAF–MEK–ERK pathway in cancer therapy. Cancer lett 283: 125–134. doi:10.1016/j.canlet.2009.01.022
Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, Kolis S, Zhao S, Lee R, Grippo JF (2010) RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res 70: 5518–5527. doi:10.1158/0008-5472.CAN-10-0646
Smith AL, DeMorin FF, Paras NA, Huang Q, Petkus JK, Doherty EM, Nixey T, Kim JL, Whittington DA, Epstein LF (2009) Selective inhibitors of the mutant B-Raf pathway: discovery of a potent and orally bioavailable aminoisoquinoline. J Med Chem 52: 6189–6192. doi:10.1021/jm901081g
Me nard D, Niculescu-Duvaz I, Dijkstra HP, Niculescu-Duvaz D, Suijkerbuijk BMJM, Zambon A, Nourry A, Roman E, Davies L, Manne HA (2009) Novel potent BRAF inhibitors: toward 1 nM compounds through optimization of the central phenyl ring. J Med Chem 52: 3881–3891. doi:10.1021/jm900242c
Zambon A, Menard D, Suijkerbuijk BMJM, Niculescu-Duvaz I, Whittaker S, Niculescu-Duvaz D, Nourry A, Davies L, Manne HA, Lopes F (2010) Novel hinge binder improves activity and pharmacokinetic properties of BRAF inhibitors. J Med Chem 53: 5639–5655. doi:10.1021/jm100383b
Lee JT, Li L, Brafford PA, VanDen Eijnden M, Halloran MB, Sproesser K, Haass NK, Smalley KSM, Tsai J, Bollag G (2010) PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E positive melanomas. Pigm Cell Melanoma Res 23: 820–827. doi:10.1111/j.1755-148X.2010.00763.x
Ramnath N, Adjei A (2007) Inhibitors of Raf kinase and MEK signaling. Update Cancer Ther 2: 111–118. doi:10.1016/j.uct.2007.10.001
Alzate-Morales JH, Vergara-Jaque A, Caballero J (2010) Computational study on the interaction of N1 substituted pyrazole derivatives with B-Raf kinase: an unusual water wire hydrogen-bond network and novel interactions at the entrance of the active site. J Chem Inf Model 50: 1101–1112. doi:10.1021/ci100049h
Liao JJL (2007) Molecular recognition of protein kinase binding pockets for design of potent and selective kinase inhibitors. J Med Chem 50: 409–424. doi:10.1021/jm0608107
Oprea TI, Marshall GR (1998) Receptor-based prediction of binding affinities. Perspect Drug Discovery Des 9(11): 35–61. doi:10.1023/A:1027299602978
Selassie C, Verma RP (2003) History of quantitative structure–activity relationships. Wiley, New York
Clark RD (2009) Prospective ligand- and target-based 3D QSAR: state of the art 2008. Curr Top Med Chem 9: 791–810. doi:10.2174/156802609789207118
Ul Haq Z, Mahmood U, Reza S, Uddin R, Aleem M (2010) Ligand based 3D QSAR studies of diaryl acyl sulfonamide analogues as human umbilical vein endothelial cells inhibitors stimulated by VEGF. Chem Biol Drug Des 77: 288–294. doi:10.1111/j.1747-0285.2011.01084.x
Cramer Iii RD, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110: 5959–5967. doi:10.1021/ja00226a005
Sheng C, Zhang W, Ji H, Zhang M, Song Y, Xu H, Zhu J, Miao Z, Jiang Q, Yao J (2006) Structure-based optimization of azole antifungal agents by CoMFA, CoMSIA, and molecular docking. J Med Chem 49: 2512–2525. doi:10.1021/jm051211n
Kubinyi H (1993) 3D QSAR in drug design: theory, methods and applications. ESCOM, Leiden
Ul Haq Z, Mahmood U, Jehangir B (2009) Ligand based 3D QSAR studies of physostigmine analogues as acetylcholinesterase inhibitors. Chem Biol Drug Des 74: 571–581. doi:10.1111/j.1747-0285.2009.00897.x
Klebe G, Abraham U (1999) Comparative molecular similarity index analysis (CoMSIA) to study hydrogen-bonding properties and to score combinatorial libraries. J Comput Aided Mol Des 13: 1–10. doi:10.1023/A:1008047919606
Klebe G, Kubinyi H, Folkers G, Martin YC (2002) Comparative molecular similarity indices analysis: CoMSIA. In: 3D QSAR in drug design. Springer, New York, pp 87–104
Niculescu-Duvaz I, Roman E, Whittaker SR, Friedlos F, Kirk R, Scanlon IJ, Davies LC, Niculescu-Duvaz D, Marais R, Springer CJ (2008) Novel inhibitors of the v-raf murine sarcoma viral oncogene homologue B1 (BRAF) based on a 2, 6-disubstituted pyrazine scaffold. J Med Chem 51: 3261–3274. doi:10.1021/jm070776b
Niculescu-Duvaz D, Gaulon C, Dijkstra HP, Niculescu-Duvaz I, Zambon A, Me nard D, Suijkerbuijk BMJM, Nourry A, Davies L, Manne H (2009) Pyridoimidazolones as novel potent inhibitors of v-Raf murine sarcoma viral oncogene homologue B1 (BRAF). J Med Chem 52: 2255–2264. doi:10.1021/jm801509w
Shih KC, Lin CY, Zhou J, Chi HC, Chen TS, Wang CC, Tseng HW, Tang CY (2011) Development of novel 3D-QSAR combination approach for screening and optimizing B-Raf inhibitors in silico. J Chem Inf Model 51: 398–407. doi:10.1021/ci100351s
Yang Y, Qin J, Liu H, Yao X (2011) Molecular dynamics simulation, free energy calculation and structure-based 3D-QSAR studies of B-RAF kinase inhibitors. J Chem Inf Model 51: 680–692. doi:10.1021/ci100427j
SYBYL Software (2002) Version 7.3. Tripos Associates, St. Louis
Clark M, Cramer Iii RD, Van Opdenbosch N (1989) Validation of the general purpose Tripos 5.2 force field. J Comput Chem 10: 982–1012. doi:10.1002/jcc.540100804
Jakalian A, Bush BL, Jack DB, Bayly CI (2000) Fast, efficient generation of high quality atomic Charges. AM1 BCC model: I. Method. J Comput Chem 21: 132–146. doi:10.1002/(SICI)1096-987X(20000130)21:2<132::AID-JCC5>3.0.CO;2-P
Jain AN (2003) Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J Med Chem 46: 499–511. doi:10.1021/jm020406h
Jain AN (2007) Surflex-dock 2.1: robust performance from ligand energetic modeling, ring flexibility, and knowledge-based search. J Comput Aided Mol Des 21: 281–306. doi:10.1007/s10822-007-9114-2
Miteva MA, Lee WH, Montes MO, Villoutreix BO (2005) Fast structure-based virtual ligand screening combining FRED, DOCK, and Surflex. J Med Chem 48: 6012–6022. doi:10.1021/jm050262h
Verma J, Khedkar VM, Coutinho EC (2010) 3D-QSAR in drug design-a review. Curr Top Med Chem 10: 95–115. doi:10.2174/156802610790232260
Tervo AJ, Nyrönen TH, Rönkkö T, Poso A (2004) Comparing the quality and predictiveness between 3D QSAR models obtained from manual and automated alignment. J Chem Inf Comput Sci 44: 807–816. doi:10.1021/ci0342268
Tropsha A, Gramatica P, Gombar VK (2003) The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb Sci 22: 69–77. doi:10.1002/qsar.200390007
Rucker C, Rucker G, Meringer M (2007) y-Randomization and its variants in QSPR/QSAR. J Chem Inf Model 47: 2345–2357. doi:10.1021/ci700157b
Mouchlis VD, Melagraki G, Mavromoustakos TM, Kollias G, Afantitis A (2012) Molecular modeling on pyrimidine-urea inhibitors of TNF- I ± production: an integrated approach using a combination of molecular docking, classification techniques and 3D-QSAR CoMSIA. J Chem Inf Model 52: 711–723. doi:10.1021/ci200579f
Zhang S, Golbraikh A, Oloff S, Kohn H, Tropsha A (2006) A novel automated lazy learning QSAR (ALL-QSAR) approach: method development, applications, and virtual screening of chemical databases using validated ALL-QSAR models. J Chem Inf Model 46: 1984–1995. doi:10.1021/ci060132x
Alexander G, Alexander T (2002) Beware of Q2. J Mol Graph Model 20: 269–276. doi:10.1016/S1093-3263(01)00123-1
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
The Below is the Electronic Supplementary Material.
Rights and permissions
About this article
Cite this article
Ul-Haq, Z., Mahmood, U. & Reza, S. A combined 3D-QSAR and molecular docking strategy to understand the binding mechanism of V600EB-RAF inhibitors. Mol Divers 16, 771–785 (2012). https://doi.org/10.1007/s11030-012-9395-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-012-9395-9