Abstract
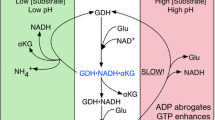
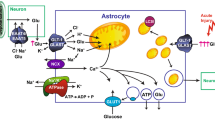
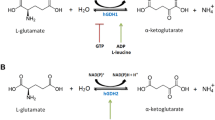
Glutamate dehydrogenase (GDH) uses ammonia to reversibly convert α-ketoglutarate to glutamate using NADP(H) and NAD(H) as cofactors. While GDH in most mammals is encoded by a single GLUD1 gene, humans and other primates have acquired a GLUD2 gene with distinct tissue expression profile. The two human isoenzymes (hGDH1 and hGDH2), though highly homologous, differ markedly in their regulatory properties. Here we obtained hGDH1 and hGDH2 in recombinant form and studied their Km for ammonia in the presence of 1.0 mM ADP. The analyses showed that lowering the pH of the buffer (from 8.0 to 7.0) increased the Km for ammonia substantially (hGDH1: from 12.8 ± 1.4 mM to 57.5 ± 1.6 mM; hGDH2: from 14.7 ± 1.6 mM to 62.2 ± 1.7 mM), thus essentially precluding reductive amination. Moreover, lowering the ADP concentration to 0.1 mM not only increased the K0.5 [NH4 +] of hGDH2, but also introduced a positive cooperative binding phenomenon in this isoenzyme. Hence, intra-mitochondrial acidification, as occurring in astrocytes during glutamatergic transmission should favor the oxidative deamination of glutamate. Similar considerations apply to the handling of glutamate by the proximal convoluted tubules of the kidney during systemic acidosis. The reverse could apply for conditions of local or systemic hyperammonemia or alkalosis.


Similar content being viewed by others
References
Adeva MM, Souto G, Blanco N, Donapetry C (2012) Ammonium metabolism in humans. Metabolism 61:1495–1511
Azarias G, Perreten H, Lengacher S, Poburko D, Demaurex N, Magistretti PJ, Chatton JY (2011) Glutamate transport decreases mitochondrial pH and modulates oxidative metabolism in astrocytes. J Neurosci 31:3550–3559
Bailey J, Bell ET, Bell JE (1982) Regulation of bovine glutamate dehydrogenase. The effects of pH and ADP. J Biol Chem 257:5579–5583
Balut C, vande Ven M, Despa S, Lambrichts I, Ameloot M, Steels P, Smets I (2008) Measurement of cytosolic and mitochondrial pH in living cells during reversible metabolic inhibition. Kidney Int 73:226–232
Cohen NS, Kyan FS, Kyan SS, Cheung CW, Raijman L (1985) The apparent Km of ammonia for carbamoyl phosphate synthetase (ammonia) in situ. Biochem J 229:205–211
Cooper A (2012) The role of glutamine synthetase and glutamate dehydrogenase in cerebral ammonia homeostasis. Neurochem Res 37:2439–2455
Engel P (1981) More than one substrate. In: Engel P (ed) Enzyme kinetics, the steady state approach. Chapmann Hall, London
Faff-Michalak L, Albrecht J (1993) Hyperammonemia and hepatic encephalopathy stimulate rat cerebral synaptic mitochondrial glutamate dehydrogenase activity specifically in the direction of glutamate oxidation. Brain Res 618:299–302
Hudson R, Daniel R (1993) L-glutamate dehydrogenases: distribution, properties and mechanism. Comp Biochem Physiol B 106:767–792
Hutson SM, Islam MM, Zaganas I (2011) Interaction between glutamate dehydrogenase (GDH) and l-leucine catabolic enzymes: Intersecting metabolic pathways. Neurochem Int 59:518–524
Kimelberg H, Vitarella D, Aschner M (1996) Effects of toxins on glia in brain edema. In: Aschner M, Kimelberg H (eds) The role of glia in neurotoxicity. CRC Press, Boca Raton, pp 311–334
Leke R, Bak L, Anker M, Melo TM, Sørensen M, Keiding S, Vilstrup H, Ott P, Portela LV, Sonnewald U, Schousboe A, Waagepetersen HS (2011) Detoxification of ammonia in mouse cortical GABAergic cell cultures increases neuronal oxidative metabolism and reveals an emerging role for release of glucose-derived alanine. Neurotox Res 19:496–510
Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY (1998) Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci 95:6803–6808
Marcaggi P, Coles JA (2001) Ammonium in nervous tissue: transport across cell membranes, fluxes from neurons to glial cells, and role in signalling. Progr Neurobiol 64:157–183
McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393
Metelkin E, Demin O, Kovacs Z, Chinopoulos C (2009) Modeling of ATP-ADP steady-state exchange rate mediated by the adenine nucleotide translocase in isolated mitochondria. FEBS J 276:6942–6955
Murthy MS, Pande SV (1985) Microcompartmentation of transported carnitine, acetylcarnitine and ADP occurs in the mitochondrial matrix. Implications for transport measurements and metabolism. Biochem J 230:657–663
Plaitakis A, Zaganas I (2001) Regulation of human glutamate dehydrogenases: implications for glutamate, ammonia and energy metabolism in brain. J Neurosci Res 66:899–908
Rama Rao KV, Jayakumar AR, Norenberg DM (2003) Ammonia neurotoxicity: role of the mitochondrial permeability transition. Metab Brain Dis 18:113–127
Schoolwerth AC, LaNoue KF, Hoover WJ (1984) Effect of pH on glutamate efflux from rat kidney mitochondria. Am J Physiol - Ren Physiol 246:F266–F271
Shashidharan P, Clarke DD, Ahmed N, Moschonas N, Plaitakis A (1997) Nerve tissue-specific human glutamate dehydrogenase that is thermolabile and highly regulated by ADP. J Neurochem 68:1804–1811
Sonnewald U, Westergaard N, Schousboe A (1997) Glutamate transport and metabolism in astrocytes. Glia 21:56–63
Swain M, Butterworth R, Blei A (1992) Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 15:449–453
Tsacopoulos M, Poitry-Yamate C, Poitry S (1997) Ammonium and glutamate released by neurons are signals regulating the nutritive function of a glial cell. J Neurosci 17:2383–2390
Vetterli L, Carobbio S, Pournourmohammadi S, Martin-Del-Rio R, Skytt DM, Waagepetersen HS, Tamarit-Rodriguez J, Maechler P (2012) Delineation of glutamate pathways and secretory responses in pancreatic islets with beta-cell specific abrogation of the glutamate dehydrogenase. Mol Biol Cell 23:3851–3862
Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A (2000) A possible role of alanine for ammonia transfer between astrocytes and glutamatergic neurons. J Neurochem 75:471–479
Wiederkehr A, Park K-S, Dupont O, Demaurex N, Pozzan T, Cline GW, Wollheim CB (2009) Matrix alkalinization: a novel mitochondrial signal for sustained pancreatic β-cell activation. EMBO J 28:417–428
Williamson DH, Lund P, Krebs HA (1967) The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103:514–527
Yu AC, Schousboe A, Hertz L (1982) Metabolic fate of 14C-labeled glutamate in astrocytes in primary culture. J Neurochem 39:954–960
Zaganas I, Plaitakis A (2002) Single amino acid substitution (G456A) in the vicinity of the GTP binding domain of human housekeeping glutamate dehydrogenase markedly attenuates GTP inhibition and abolishes the cooperative behavior of the enzyme. J Biol Chem 277:26422–26428
Zaganas I, Spanaki C, Plaitakis A (2012) Expression of human GLUD2 glutamate dehydrogenase in human tissues: functional implications. Neurochem Int 61:455–462
Acknowledgments
This research has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program: THALIS –UOC, Title “Mitochondrial dysfunction in neurodegenerative diseases” (MDND grant, University of Crete Code 3578). Also, support was obtained from the “Association for research and treatment of neurologic diseases of Crete-EY ZHN”, the Lundbeck Foundation and The Danish Medical Research Council 09-066319.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ioannis Zaganas and Kamilla Pajęcka contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zaganas, I., Pajęcka, K., Wendel Nielsen, C. et al. The effect of pH and ADP on ammonia affinity for human glutamate dehydrogenases. Metab Brain Dis 28, 127–131 (2013). https://doi.org/10.1007/s11011-013-9382-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-013-9382-6