Abstract
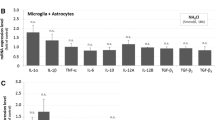
Brain edema, a lethal complication of acute liver failure (ALF), is believed to be largely cytotoxic due to the swelling of astrocytes. Ammonia, a principal neurotoxin in ALF, has been strongly implicated in the development of the brain edema. It was previously shown that treatment of cultured astrocytes with ammonia (5 mM NH4Cl) results in cell swelling. While ammonia continues to exert a direct effect on astrocytes, it is possible that ammonia can affect other neural cells, particularly microglia. Microglia are capable of evoking an inflammatory response, a process known to contribute to the brain edema. We therefore examined the potential role of microglia in the mechanism of ammonia-induced astrocyte swelling. Conditioned media (CM) derived from ammonia-treated cultured microglia when added to cultured astrocytes resulted in significant cell swelling. Such swelling was synergistically increased when astrocytes were additionally treated with 5 mM ammonia. CM from ammonia-treated microglia also showed significant release of oxy-radicals and nitric oxide into the CM. CM from ammonia-treated microglia containing Tempol (a superoxide scavenger) or uric acid (a peroxynitrite scavenger) when added to astrocytes resulted in marked reduction in the cell swelling. Together, these studies indicate that microglia contribute to the ammonia-induced astrocyte swelling by a mechanism involving oxidative/nitrosative stress.



Similar content being viewed by others
References
Ducis I, Norenberg LO, Norenberg MD (1990) The benzodiazepine receptor in cultured astrocytes from genetically epilepsy-prone rats. Brain Res 531:318–321
Flanary BE, Streit WJ (2004) Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia 45:75–88
Garden GA, Moller T (2006) Microglia biology in health and disease. J Neuroimm Pharmacol 1:127–137
Gregorios JB, Mozes LW, Norenberg LO, Norenberg MD (1985) Morphologic effects of ammonia on primary astrocyte cultures. I. light microscopic studies. J Neuropathol Exp Neurol 44:397–403
Grisham MB, Johnson GG, Lancaster JR (1996) Quantitation of nitrate and nitrite in extracellular fluids. Meth Enzymol 268:237–246
Grzelak A, Rychlik B, Bartosz G (2001) Light-dependent generation of reactive oxygen species in cell culture media. Free Rad Biol Med 30:1418–1425
Jayakumar AR, Valdes V, Norenberg MD (2011) The Na-K-Cl co-transporter in the brain edema in acute liver failure. J Hepatol 54:272–280
Jayakumar AR, Tong XY, Ospel J, Norenberg MD (2012) Role of cerebral endothelial cells in astrocyte swelling and brain edema associated with acute hepatic encephalopathy. Neuroscience 218:305–316
Jiang W, Desjardin P, Butterworth RF (2009) Direct inflammation contributes to encephalopathy and brain edema in acute liver failure. J Neurochem 109:485–493
Jones EA, Weissenborn K (1997) Neurology and the liver. J Neurol Neurosurg Psychiatr 63:279–293
Kletzien RF, Pariza MW, Becker JE, Potter VR (1975) A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem 68:537–544
Mans AM, Saunders SJ, Kirsch RE, Biebuyck JF (1979) Correlation of plasma and brain amino acid and putative neurotransmitter alterations during acute hepatic coma in the rat. J Neurochem 32:285–292
Mans AM, DeJoseph MR, Hawking RA (1994) Metabolic abnormalities and grade of encephalopathy in acute hepatic failure. J Neurochem 63:1829–1838
Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT (1991) Ammonia-induced astrocyte swelling in primary culture. Neurochem Res 16:833–836
Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS (2007) New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 22:219–234
Pontén U, Ratcheson RA, Salford LG, Siesjö BK (1973) Optimal freezing conditions for cerebral metabolites in rats. J Neurochem 21:1127–1138
Rama Rao KV, Reddy PVB, Tong X, Norenberg MD (2010) Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol 176:1400–1408
Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo JM, Felipo V (2010) Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139:675–684
Swain M, Butterworth RF, Blei AT (1992) Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 15:449–453
Traber PG, Dal Canto MC, Ganger D, Blei AT (1987) Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology 7:1272–1277
Wilkinson SP, Arroyo V, Moodie H, Williams R (1974) Proceedings: endotoxaemia in fulminant hepatic failure. Clin Sci Mol Med 46:30P–31P
Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z, Williams R, Davies C, Moore KP, Jalan R (2007) Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology 45:1517–1526
Zemtsova I, Görg B, Keitel V, Bidmon HJ, Schror K, Haüssinger D (2011) Microglia activation in hepatic encephalopathy in rats and humans. Hepatology 54:204–215
Acknowledgments
This work was supported by a VA Merit Review and NIH grant (DK063311). We are grateful to Alina Fernandez for the preparation of astrocyte and microglia cultures, and to Dr. A.R. Jayakumar for measurements of reactive oxygen and nitrogen species.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rao, K.V.R., Brahmbhatt, M. & Norenberg, M.D. Microglia contribute to ammonia-induced astrocyte swelling in culture. Metab Brain Dis 28, 139–143 (2013). https://doi.org/10.1007/s11011-012-9339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9339-1