Abstract
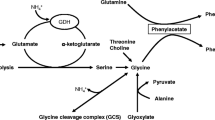
Glutamine synthetase (GS) is highly active in astrocytes, and these cells are physiologically and morphologically compromised by hyperammonemia. Hyperammonemia in end-stage acute liver failure (ALF) is often associated with cerebral edema and astrocyte pathology/swelling. Many studies of animal models of hyperammonemia, and, more recently, nuclear magnetic resonance studies of liver disease patients, have shown that cerebral glutamine is elevated in hyperammonemia, contributing to the edema and encephalopathy. The GS inhibitor L-methionine-S,R-sulfoximine (MSO) is protective in animal models against acute ammonia intoxication. MSO is also an inhibitor of glutamate cysteine ligase, is converted to metabolic products, and causes convulsions at high doses. However, the susceptibility to MSO-induced convulsions is species dependent, with primates being relatively resistant. Moreover, it is possible to chronically maintain cerebral GS activity in mice at low levels by MSO treatment without any obvious untoward effects. Furthermore, MSO is protective in a mouse model of ALF. Extreme caution would be needed in administering MSO to patients. Nevertheless, inhibition of brain GS by MSO (or other GS inhibitors) may have therapeutic benefit in ALF.


Similar content being viewed by others
Notes
Ammonia free base (NH3) has a pK a of ~9.2. Thus, under normal intracellular physiological conditions (pH 7.2 – 7.4) ammonia exists predominantly (~99 %) as the conjugate acid, ammonium (NH +4 ). For convenience, ammonia is used throughout the text to indicate the sum of NH3 plus NH +4 .
Commercially available MSO exists as a pair of diastereoisomers (L,S and L,R). The L,S diastereoisomer is a potent GS inhibitor, whereas the L,R diastereoisomer is not (Rowe and Meister 1970). In some studies, as noted in the text, a mixture of D,L-methionine-S,R-sulfoximine isomers was used.
References
Bentley HR, McDermott EE, Whitehead JK (1951) Action of nitrogen trichloride on certain proteins. II. Synthesis of methionine sulphoximine and other sulphoximines. Proc R Soc Lond B Biol Sci 138(891):265–272
Blin M, Crusio WE, Hévor T, Cloix J-F (2002) Chronic inhibition of glutamine synthetase is not associated with impairment of learning and memory in mice. Brain Res Bull 57:11–15
Brosnan ME, Brosnan JT (2009) Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 90:857S–861S
Brusilow SW, Koehler RC, Traystman RJ, Cooper AJL (2010) Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics 7:452–470
Chatauret N, Desjardins P, Zwingmann C, Rose C, Rao KV, Butterworth RF (2006) Direct molecular and spectroscopic evidence for increased ammonia removal capacity of skeletal muscle in acute liver failure. J Hepatol 44:1083–1088
Chavarria L, Alonso J, Rovira A, Córdoba J (2011) Neuroimaging in acute liver failure. Neurochem Int 59:1175–1180
Cooper AJL (2012) The role of glutamine synthetase and glutamate dehydrogenase in cerebral ammonia homeostasis. Neurochem Res. [Epub ahead of print]
Cooper AJL, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Cooper AJL, Stephani RA, Meister A (1976) Enzymatic reactions of methionine sulfoximine. Conversion to the corresponding α-imino and α-keto acids and to α-ketobutyrate and methane sulfinimide. J Biol Chem 251:6674–6682
Cooper AJL, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE (1979) The metabolic fate of 13N-labeled ammonia in rat brain. J Biol Chem 254:4982–4992
Cooper AJL, Vergara F, Duffy TE (1983) Cerebral glutamine metabolism. In: Hertz L, Kvamme E, McGeer EG, Schousboe A (eds) Glutamine, glutamate and GABA in the central nervous system. Alan R Liss, Inc, New York, pp 77–93
Cooper AJL, Mora SN, Cruz NF, Gelbard AS (1985) Cerebral ammonia metabolism in hyperammonemic rats. J Neurochem 44:1716–1723
Desjardins P, Du T, Jiang W, Peng L, Butterworth RF (2012) Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: Role of glutamine redefined. Neurochem Int 60:690–696
Duarte JMN, Lanz B, Gruetter R (2011) Compartmentalised cerebral metabolism of [1,6-13C]glucose determined by in vivo 13C NMR spectroscopy at 14.1T. Front Neuroenergetics 3:3
Folbergrová J, Passonneau JV, Lowry OH, Schulz DW (1969) Glycogen, ammonia and related metabolities in the brain during seizures evoked by methionine sulphoximine. J Neurochem 16:191–203
Gershoff SN, Elvehjem CA (1951) The relative effect of methionine sulfoximine on different animal species. J Nutr 45:451–458
Griffith OW, Meister A (1978) Differential inhibition of glutamine and γ-glutamylcysteine synthetases by α-alkyl analogs of methionine sulfoximine that induce convulsions. J Biol Chem 253:2333–2338
Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254:7558–7560
Hawkins RA, Jessy J, Mans AM, De Joseph MR (1993) Effect of reducing brain glutamine synthesis on metabolic symptoms of hepatic encephalopathy. J Neurochem 60:1000–1006
Jambekar AA, Palma E, Nicolosi L, Rasola A, Petronilli V, Chiara F, Bernardi P, Needleman R, Brusilow WS (2011) A glutamine synthetase inhibitor increases survival and decreases cytokine response in a mouse model of acute liver failure. Liver Int 31:1209–1221
Keiding S, Sørensen M, Bender D, Munk OL, Ott P, Vilstrup H (2006) Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology 43:42–50
Krakoff IH (1961) Effect of methionine sulfoximine in man. Clin Pharmacol Ther 2:599–604
Mans AM, Saunders SJ, Kirsch RE, Biebuyck JF (1979) Correlation of plasma and brain amino acid and putative neurotransmitter alterations during acute hepatic coma in the rat. J Neurochem 32:285–292
Mans AM, DeJoseph MR, Hawkins RA (1994) Metabolic abnormalities and grade of encephalopathy in acute hepatic failure. J Neurochem 63:1829–1838
Meister A (1980) Catalytic mechanism of glutamine synthetase; overview of glutamine metabolism. In: Mora J, Palacios R (eds) Glutamine metabolism, enzymology, and regulation. Academic Press, New York, pp 1–40
Mellanby E (1946) Diet and canine hysteria; experimental production by treated flour. Br Med J 2(4484):885–887
Newell GW, Erickson TC, Gilson WE, Gershoff SN, Elvehjem CA (1949) Studies on human subjects receiving highly agenized food materials. J Lab Clin Med 34:239–245
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161:303–310
Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, Arroliga AC, Mullen KD (2003) Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 114:188–193
Pace J, McDermott E (1952) Methionine sulphoximine and some enzyme systems involving glutamine. Nature 169:415–416
Pamiljans V, Krishnaswamy PR, Dumville G, Meister A (1962) Studies on the mechanism of glutamine synthesis; Isolation and properties of the enzyme from sheep brain. Biochemistry 1:153–158
Pollock GH (1949) Species specificity of agene toxicity. J Appl Physiol 1:802–806
Pontén U, Ratcheson RA, Salford LG, Siesjö BK (1973) Optimal freezing conditions for cerebral metabolites in rats. J Neurochem 21:1127–1138
Rowe WB, Meister A (1970) Identification of L-methionine-S-sulfoximine as the convulsant isomer of methionine sulfoximine. Proc Natl Acad Sci U S A 66:500–506
Sellinger OZ, Weliler P Jr (1963) the nature of the inhibition in vitro of cerebral glutamine synthetase by the convulsant, methionine sulfoximine. Biochem Pharmacol 12:989–1000
Sellinger OZ, Schatz RA, Gregor P (1986) Cerebral methylations in epileptogenesis. Adv Neurol 44:465–473
Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG (2001) In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during [2-13C]glucose infusion. J Neurochem 76:975–989
Veech RL, Harris RL, Veloso D, Veech EH (1973) Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem 20:183–188
Warren KS, Schenker S (1964) Effect of an inhibitor of glutamine synthesis (methionine sulfoximine) on ammonia toxicity and metabolism. J Lab Clin Med 64:442–449
Acknowledgments
I thank Dr. Saul Brusilow for his help and encouragement and for providing me with some pertinent references regarding the history of MSO. Dr. Brusilow has been a major proponent for the possible use of a GS inhibitor in the treatment of ALF. I also thank Dr. Boris F. Krasnikov for help in construction of the figures. Some of the author’s work mentioned in this review was supported by NIH grant DK 16739.
Conflict of interest
No potential conflict of interest relevant to this article is reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cooper, A.J.L. Possible treatment of end-stage hyperammonemic encephalopathy by inhibition of glutamine synthetase. Metab Brain Dis 28, 119–125 (2013). https://doi.org/10.1007/s11011-012-9338-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9338-2