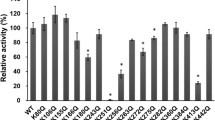
Abstract
The carnitine/acylcarnitine transporter (CACT; SLC25A20) mediates an antiport reaction allowing entry of acyl moieties in the form of acylcarnitines into the mitochondrial matrix and exit of free carnitine. The transport function of CACT is crucial for the β-oxidation pathway. In this work, it has been found that CACT is partially acetylated in rat liver mitochondria as demonstrated by anti-acetyl-lys antibody immunostaining. Acetylation was reversed by the deacetylase Sirtuin 3 in the presence of NAD+. After treatment of the mitochondrial extract with the deacetylase, the CACT activity, assayed in proteoliposomes, increased. The half-saturation constant of the CACT was not influenced, while the V max was increased by deacetylation. Sirtuin 3 was not able to deacetylate the CACT when incubation was performed in intact mitoplasts, indicating that the acetylation sites are located in the mitochondrial matrix. Prediction on the localization of acetylated residues by bioinformatics correlates well with the experimental data. Recombinant CACT treated with acetyl-CoA was partially acetylated by non-enzymatic mechanism with a corresponding decrease of transport activity. The experimental data indicate that acetylation of CACT inhibits its transport activity, and thus may contribute to the regulation of the mitochondrial β-oxidation pathway.






Similar content being viewed by others
Abbreviations
- SIRT:
-
Sirtuin
- BSA:
-
Bovine serum albumin
- PTM:
-
Post-translational modification
- CACT:
-
Carnitine/acylcarnitine transporter
- LCAD:
-
Long-chain acyl-CoA dehydrogenase
- CPT1:
-
Carnitine palmitoyltransferase I
- CAT:
-
Carnitine O-acetyltransferase
- GSH:
-
l-Glutathione reduced
- GSSG:
-
l-Glutathione oxidized
- AAC:
-
ADP/ATP carrier
- NAM:
-
Nicotinamide
- PVDF:
-
Polyvinyldifluoride membrane
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NEM:
-
N-Ethylmaleimide
References
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327:1000–1004. doi:10.1126/science.1179689
Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP (2010) Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327:1004–1007. doi:10.1126/science.1179687
Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23:607–618. doi:10.1016/j.molcel.2006.06.026
Rauh D, Fischer F, Gertz M, Lakshminarasimhan M, Bergbrede T, Aladini F, Kambach C, Becker CF, Zerweck J, Schutkowski M, Steegborn C (2013) An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat Commun 4:2327. doi:10.1038/ncomms3327
Weinert BT, Schölz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, Choudhary C (2013) Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep 4:842–851. doi:10.1016/j.celrep.2013.07.024
Palmieri EM, Spera I, Menga A, Infantino V, Porcelli V, Iacobazzi V, Pierri CL, Hooper DC, Palmieri F, Castegna A (2015) Acetylation of human mitochondrial citrate carrier modulates mitochondrial citrate/malate exchange activity to sustain NADPH production during macrophage activation. Biochim Biophys Acta 1847:729–738. doi:10.1016/j.bbabio.2015.04.009
Davies MN, Kjalarsdottir L, Thompson JW, Dubois LG, Stevens RD, Ilkayeva OR, Brosnan MJ, Rolph TP, Grimsrud PA, Muoio DM (2016) The acetyl group buffering action of carnitine acetyltransferase offsets macronutrient-induced lysine acetylation of mitochondrial proteins. Cell Rep 14:243–254. doi:10.1016/j.celrep.2015.12.030
Pougovkina O, te Brinke H, Ofman R, van Cruchten AG, Kulik W, Wanders RJ, Houten SM, de Boer VC (2014) Mitochondrial protein acetylation is driven by acetyl-CoA from fatty acid oxidation. Hum Mol Genet 23:3513–3522. doi:10.1093/hmg/ddu059
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Alt FW, Kahn CR, Verdin E (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464:121–125. doi:10.1038/nature08778
Giangregorio N, Palmieri F, Indiveri C (2013) Glutathione controls the redox state of the mitochondrial carnitine/acylcarnitine carrier Cys residues by glutathionylation. Biochim Biophys Acta 1830:5299–5304. doi:10.1016/j.bbagen.2013.08.003
Giangregorio N, Tonazzi A, Console L, Lorusso I, De Palma A, Indiveri C (2016) The mitochondrial carnitine/acylcarnitine carrier is regulated by hydrogen sulfide via interaction with C136 and C155. Biochim Biophys Acta 1860:20–27. doi:10.1016/j.bbagen.2015.10.005
Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E (2015) PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 43:D512–D520. doi:10.1093/nar/gku1267
Tonazzi A, Giangregorio N, Console L, Indiveri C (2015) Mitochondrial carnitine/acylcarnitine translocase: insights in structure/function relationships. Basis for drug therapy and side effects prediction. Min Rev Med Chem 15:396–405
Giangregorio N, Tonazzi A, Indiveri C, Palmieri F (2007) Conformation-dependent accessibility of Cys-136 and Cys-155 of the mitochondrial rat carnitine/acylcarnitine carrier to membrane-impermeable SH reagents. Biochim Biophys Acta 1767:1331–1339. doi:10.1016/j.bbabio.2007.08.010
Indiveri C, Iacobazzi V, Giangregorio N, Palmieri F (1998) Bacterial overexpression, purification, and reconstitution of the carnitine/acylcarnitine carrier from rat liver mitochondria. Biochem Biophys Res Commun 249:589–594. doi:10.1006/bbrc.1998.9197
Giangregorio N, Console L, Tonazzi A, Palmieri F, Indiveri C (2014) Identification of amino acid residues underlying the antiport mechanism of the mitochondrial carnitine/acylcarnitine carrier by site-directed mutagenesis and chemical labeling. Biochemistry 53:6924–6933. doi:10.1021/bi5009112
Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ, Brandolin G (2003) Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426:39–44. doi:10.1038/nature02056
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Tonazzi A, Mantovani C, Colella M, Terenghi G, Indiveri C (2013) Localization of mitochondrial carnitine/acylcarnitine translocase in sensory neurons from rat dorsal root Ganglia. Neuro Chem Res 38:2535–2541. doi:10.1007/s11064-013-1168-z
Indiveri C, Capobianco L, Krämer R, Palmieri F (1989) Kinetics of the reconstituted dicarboxylate carrier from rat liver mitochondria. Biochim Biophys Acta 977:187–193
Kaplan RS, Pedersen PL (1985) Isolation and reconstitution of the n-butylmalonate-sensitive dicarboxylate transporter from rat liver mitochondria. J Biol Chem 260:10293–10298
Scalise M, Pochini L, Giangregorio N, Tonazzi A, Indiveri C (2013) Proteoliposomes as tool for assaying membrane transporter functions and interactions with xenobiotics. Pharmaceutics 5:472–497. doi:10.3390/pharmaceutics5030472
Console L, Giangregorio N, Indiveri C, Tonazzi A (2014) Carnitine/acylcarnitine translocase and carnitine palmitoyltransferase 2 form a complex in the inner mitochondrial membrane. Mol Cell Biochem 394:307–314. doi:10.1007/s11010-014-2098-z
Verdin E, Hirschey MD, Finley LW, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35:669–675. doi:10.1016/j.tibs.2010.07.003
Giangregorio N, Tonazzi A, Console L, Indiveri C, Palmieri F (2010) Site-directed mutagenesis of charged amino acids of the human mitochondrial carnitine/acylcarnitine carrier: insight into the molecular mechanism of transport. Biochim Biophys Acta 1797:839–845. doi:10.1016/j.bbabio.2010.03.017
Tonazzi A, Giangregorio N, Indiveri C, Palmieri F (2009) Site-directed mutagenesis of the His residues of the rat mitochondrial carnitine/acylcarnitine carrier: implications for the role of His-29 in the transport pathway. Biochim Biophys Acta 1787:1009–1015. doi:10.1016/j.bbabio.2009.02.026
Tonazzi A, Console L, Indiveri C (2013) Inhibition of mitochondrial carnitine/acylcarnitine transporter by H(2)O(2): molecular mechanism and possible implication in pathophysiology. Chem Biol Interact. doi:10.1016/j.cbi.2013.01.006
Arduini A, Bonomini M, Savica V, Amato A, Zammit V (2008) Carnitine in metabolic disease: potential for pharmacological intervention. Pharmacol Ther 120:149–156. doi:10.1016/j.pharmthera.2008.08.008
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723. doi:10.1002/elps.1150181505
Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW (2013) Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci 110(16):6601–6606. doi:10.1073/pnas.1302961110
Acknowledgements
This work was supported by funds from: Programma Operativo Nazionale [01_00937] - MIUR “Modelli sperimentali biotecnologici integrati per lo sviluppo e la selezione di molecole di interesse per la salute dell’uomo”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The experiments comply with the current Italian laws.
Additional information
Nicola Giangregorio and Annamaria Tonazzi have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Giangregorio, N., Tonazzi, A., Console, L. et al. Post-translational modification by acetylation regulates the mitochondrial carnitine/acylcarnitine transport protein. Mol Cell Biochem 426, 65–73 (2017). https://doi.org/10.1007/s11010-016-2881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2881-0