Abstract
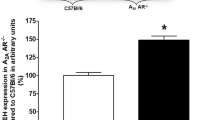
Soluble epoxide hydrolase (sEH) converts epoxyeicosatrienoic acids that are endothelium-derived hyperpolarizing factors into less active dihydroxyeicosatrienoic acids. Previously, we reported a decrease in adenosine A1 receptor (A1AR) protein levels in sEH knockout (sEH−/−) and an increase in sEH and A1AR protein levels in A2AAR−/− mice. Additionally, KATP channels are involved in adenosine receptor (AR)-dependent vascular relaxation. Thus, we hypothesize that a potential relationship may exist among sEH over-expression, A1AR upregulation, inactivation of KATP channels, and increased in vascular tone. We performed DMT myograph muscle tension measurements and western blot analysis in isolated mouse mesenteric arteries (MAs) from wild-type (WT) and endothelial over-expression of sEH (Tie2-sEH Tr) mice. Our data revealed that NECA (a non-selective adenosine receptors agonist)-induced relaxation was significantly reduced in Tie2-sEH Tr mice, and CCPA (A1AR agonist)-induced contraction was increased in Tie2-sEH Tr mice. A1AR-dependent contraction in Tie2-sEH Tr mice was significantly attenuated by pharmacological inhibition of CYP4A (HET0016, 10 µM), PKCα (GO6976, 1 µM), and ERK1/2 (PD58059, 1 µM). Our western blot analysis revealed significantly higher basal protein expression of CYP4A, A1AR, and reduced p-ERK in MAs of Tie2-sEH Tr mice. Notably, pinacidil (KATP channel opener)-induced relaxation was also significantly reduced in MAs of Tie2-sEH Tr mice. Furthermore, KATP channel-dependent relaxation in MAs was enhanced by inhibition of PKCα and ERK1/2 in WT but not Tie2-sEH Tr mice. In conclusion, our data suggest that over-expression of sEH enhances A1AR-dependent contraction and reduces KATP channel-dependent relaxation in MAs. These results suggest a possible interaction between sEH, A1AR, and KATP channels in regulating vascular tone.








Similar content being viewed by others
References
Newman JW, Morisseau C, Hammock BD (2005) Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res 44:1–51
Wang P, Meijer J, Guengerich FP (1982) Purification of human liver cytosolic epoxide hydrolase and comparison to the microsomal enzyme. Biochem 21:5769–5776
Zordoky BN, El-Kadi AO (2010) Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol Therapeutics 125:446–463
Campbell WB, Gebremedhin D, Pratt PF, Harder DR (1996) Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78:415–423
Proctor KG, Falck JR, Capdevila J (1987) Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res 60:50–59
Roman RJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82:131–185
Imig JD (2012) Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev 92:101–130
Fleming I (2007) DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol Sci 28:448–452
Fleming I (2014) The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev 66:1106–1140
Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ (2008) Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol 295:H2068–H2078
Enayetallah AE, French RA, Barber M, Grant DF (2006) Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem 54:329–335
Wang Q, Huo L, He J, Ding W, Su H, Tian D, Welch C, Hammock BD, Ai D, Zhu Y (2015) Soluble epoxide hydrolase is involved in the development of atherosclerosis and arterial neointima formation by regulating smooth muscle cell migration. Am J Physiol Heart Circ Physiol 309:H1894–H1903
Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM (2008) Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol 295:H2128–H2134
Imig JD, Walsh KA, Hye Khan MA, Nagasawa T, Cherian-Shaw M, Shaw SM, Hammock BD (2012) Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor gamma agonist improve vascular function and decrease renal injury in hypertensive obese rats. Exp Biol Med 237:1402–1412
Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, Busse R, Fleming I (2005) Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension 45:759–765
Sun D, Cuevas AJ, Gotlinger K, Hwang SH, Hammock BD, Schwartzman ML, Huang A (2014) Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol 306:H1146–H1153
Zhang W, Davis CM, Edin ML, Lee CR, Zeldin DC, Alkayed NJ (2013) Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLoS One 8:e61244
Jacobson KA, Gao ZG (2006) Adenosine receptors as therapeutic targets, Nature reviews. Drug discovery 5:247–264
Nayeem MA, Pradhan I, Mustafa SJ, Morisseau C, Falck JR, Zeldin DC (2013) Adenosine A2A receptor modulates vascular response in soluble epoxide hydrolase-null mice through CYP-epoxygenases and PPARgamma. Am J Physiol Regul Integr Comp Physiol 304:R23–R32
Ponnoth DS, Nayeem MA, Kunduri SS, Tilley SL, Zeldin DC, Ledent C, Mustafa SJ (2012) Role of omega-hydroxylase in adenosine-mediated aortic response through MAP kinase using A2A-receptor knockout mice. Am J Physiol Regul Integr Comp Physiol 302:R400–R408
Pradhan I, Ledent C, Mustafa SJ, Morisseau C, Nayeem MA (2015) High salt diet modulates vascular response in AAR and A AR mice: role of sEH, PPARgamma, and K channels. Mol Cell Biochem 404:87–96
Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, Mustafa SJ (2009) A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 297:H1032–H1039
Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ (2005) Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol 288:H1411–H1416
Kunduri SS, Dick GM, Nayeem MA, Mustafa SJ (2013) Adenosine A receptor signaling inhibits BK channels through a PKCalpha-dependent mechanism in mouse aortic smooth muscle. Physiol Rep 1:e00037
Kunduri SS, Mustafa SJ, Ponnoth DS, Dick GM, Nayeem MA (2013) Adenosine A1 receptors link to smooth muscle contraction via CYP4a, protein kinase C-alpha, and ERK1/2. J Cardiovasc Pharmacol 62:78–83
Yadav VR, Nayeem MA, Tilley SL, Mustafa SJ (2015) Angiotensin II stimulation alters vasomotor response to adenosine in mouse mesenteric artery: role for A1 and A2B adenosine receptors. Br J Pharmacol 172:4959–4969
Rodrigo GC, Standen NB (2005) ATP-sensitive potassium channels. Curr Pharm Des 11:1915–1940
Aziz Q, Thomas AM, Khambra T, Tinker A (2012) Regulation of the ATP-sensitive potassium channel subunit, Kir6.2, by a Ca2+-dependent protein kinase C. J Biol Chem 287:6196–6207
Ponnoth DS, Nayeem MA, Tilley SL, Ledent C, Mustafa SJ (2012) CYP-epoxygenases contribute to A2A receptor-mediated aortic relaxation via sarcolemmal KATP channels. Am J Physiol Regul Integr Comp Physiol 303:R1003–R1010
Teng B, Fil D, Tilley SL, Ledent C, Krahn T, Mustafa SJ (2013) Functional and RNA expression profile of adenosine receptor subtypes in mouse mesenteric arteries. J Cardiovasc Pharmacol 61:70–76
Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN (1997) Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. PNAS 94:3058–3063
Ihara E, Yu Q, Chappellaz M, MacDonald JA (2015) ERK and p38MAPK pathways regulate myosin light chain phosphatase and contribute to Ca2+ sensitization of intestinal smooth muscle contraction. Neurogastroenterol Motil 27:135–146
Ok SH, Kwon SC, Yeol Han J, Yu J, Shin IW, Lee HK, Chung YK, Choi MJ, Sohn JT (2014) Mepivacaine-induced contraction involves increased calcium sensitization mediated via Rho kinase and protein kinase C in endothelium-denuded rat aorta. Eur J Pharmacol 723:185–193
Anderson NG, Maller JL, Tonks NK, Sturgill TW (1990) Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343:651–653
Zhao Y, Zhang L, Longo LD (2005) PKC-induced ERK1/2 interactions and downstream effectors in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol 289:R164–R171
Armstead WM, Riley J, Cines DB, Higazi AA (2011) tPA contributes to impairment of ATP and Ca sensitive K channel mediated cerebrovasodilation after hypoxia/ischemia through upregulation of ERK MAPK. Brain Res 1376:88–93
Zhang DM, Chai Y, Erickson JR, Brown JH, Bers DM, Lin YF (2014) Intracellular signalling mechanism responsible for modulation of sarcolemmal ATP-sensitive potassium channels by nitric oxide in ventricular cardiomyocytes. J Physiol 592:971–990
Panhwar F, Rainbow RD, Jackson R, Davies NW (2015) Ca2+ dependent but PKC independent signalling mediates UTP induced contraction of rat mesenteric arteries. J Smooth Muscle Res 51:58–69
Cain AE, Tanner DM, Khalil RA (2002) Endothelin-1–induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca(2+)](i) sensitization pathways. Hypertension 39:543–549
Acknowledgments
This study was supported by HL-114559 (to Mohammed Nayeem, PhD.) and z01-ES025034 (Darryl Zeldin, M.D.). The authors would like to thank Jamal Mustafa, PhD. for allowing Vishal Yadav, PhD. (Postdoctoral Fellow in his lab) to work in this project. The authors appreciate the help provided by Sherry Xie for western blot experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, V.R., Hong, K.L., Zeldin, D.C. et al. Vascular endothelial over-expression of soluble epoxide hydrolase (Tie2-sEH) enhances adenosine A1 receptor-dependent contraction in mouse mesenteric arteries: role of ATP-sensitive K+ channels. Mol Cell Biochem 422, 197–206 (2016). https://doi.org/10.1007/s11010-016-2821-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2821-z